Nanosponges: An overlooked promising strategy to combat SARS-CoV-2
- PMID: 35908684
- PMCID: PMC9330373
- DOI: 10.1016/j.drudis.2022.07.015
Nanosponges: An overlooked promising strategy to combat SARS-CoV-2
Abstract
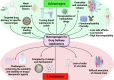
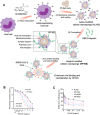
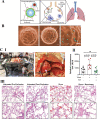
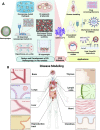
Among explored nanomaterials, nanosponge-based systems have exhibited inhibitory effects for the biological neutralization of, and antiviral delivery against, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). More studies could pave the path for clarification of their biological neutralization mechanisms as well as the assessment of their long-term biocompatibility and biosafety issues before clinical translational studies. In this review, we discuss recent advances pertaining to antiviral delivery and inhibitory effects of nanosponges against SARS-CoV-2, focusing on important challenges and opportunities. Finally, as promising approaches for recapitulating the complex structure of different organs/tissues of the body, we discuss the use of 3D in vitro models to investigate the mechanism of SARS-CoV-2 infection and to find therapeutic targets to better manage and eradicate coronavirus 2019 (COVID-19).
Keywords: Antiviral delivery; Biological neutralization; COVID-19; Nanosponges; SARS-CoV-2; Viral inactivation.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Surface Glycan Modification of Cellular Nanosponges to Promote SARS-CoV-2 Inhibition.J Am Chem Soc. 2021 Oct 27;143(42):17615-17621. doi: 10.1021/jacs.1c07798. Epub 2021 Oct 14. J Am Chem Soc. 2021. PMID: 34647745
-
Current status of antivirals and druggable targets of SARS CoV-2 and other human pathogenic coronaviruses.Drug Resist Updat. 2020 Dec;53:100721. doi: 10.1016/j.drup.2020.100721. Epub 2020 Aug 26. Drug Resist Updat. 2020. PMID: 33132205 Free PMC article. Review.
-
Antiviral Efficacies of FDA-Approved Drugs against SARS-CoV-2 Infection in Ferrets.mBio. 2020 May 22;11(3):e01114-20. doi: 10.1128/mBio.01114-20. mBio. 2020. PMID: 32444382 Free PMC article.
-
Antimicrobial peptides: A promising tool to combat multidrug resistance in SARS CoV2 era.Microbiol Res. 2022 Dec;265:127206. doi: 10.1016/j.micres.2022.127206. Epub 2022 Sep 21. Microbiol Res. 2022. PMID: 36162150 Free PMC article. Review.
-
Recent advances in potential drug therapies combating COVID-19 and related coronaviruses-A perspective.Food Chem Toxicol. 2021 Aug;154:112333. doi: 10.1016/j.fct.2021.112333. Epub 2021 Jun 10. Food Chem Toxicol. 2021. PMID: 34118347 Free PMC article. Review.
Cited by
-
Nanosponge: A promising and intriguing strategy in medical and pharmaceutical Science.Heliyon. 2023 Dec 6;10(1):e23303. doi: 10.1016/j.heliyon.2023.e23303. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38163139 Free PMC article. Review.
-
State-of-art high-performance Nano-systems for mutated coronavirus infection management: From Lab to Clinic.OpenNano. 2022 Nov-Dec;8:100078. doi: 10.1016/j.onano.2022.100078. Epub 2022 Sep 10. OpenNano. 2022. PMID: 40477696 Free PMC article. Review.
-
In vitro and preclinical evaluation of the antifungal activity of 6-methoxy-1 H-indole-2-carboxylic acid produced by Bacillus toyonensis strain OQ071612 formulated as nanosponge hydrogel.Microb Cell Fact. 2025 Apr 1;24(1):77. doi: 10.1186/s12934-025-02688-y. Microb Cell Fact. 2025. PMID: 40169999 Free PMC article.
-
Nanosponge hydrogel of octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl) propanoate of Alcaligenes faecalis.Appl Microbiol Biotechnol. 2024 Dec;108(1):100. doi: 10.1007/s00253-023-12819-3. Epub 2024 Jan 12. Appl Microbiol Biotechnol. 2024. PMID: 38217256 Free PMC article.
References
-
- Iravani S., Varma R.S. Important roles of oligo- and polysaccharides against SARS-CoV-2: recent advances. App Sci. 2021;11:3512.
-
- Jain V.P., Chaudhary S., Sharma D., Dabas N., Lalji R.S.K., Singh B.K., et al. Advanced functionalized nanographene oxide as a biomedical agent for drug delivery and anti-cancerous therapy: a review. Eur Polym J. 2021;142:110124.
-
- Jamalipour Soufi G., Iravani P., Hekmatnia A., Mostafavi E., Khatami M., Iravani S. MXenes and MXene-based materials with cancer diagnostic applications: challenges and opportunities. Comments on Inorg Chem. 2021;42(3):174–207.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

