Malignant oligoastrocytoma in the spinal cord of a cat
- PMID: 35908858
- PMCID: PMC9523307
- DOI: 10.1292/jvms.22-0144
Malignant oligoastrocytoma in the spinal cord of a cat
Abstract
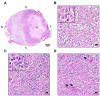
A 12-year and 3-month spayed female mixed cat was presented with severe lumbar pain. Magnetic resonance imaging and postmortem examination revealed a swollen lesion in the spinal cord at L3 level. Histologic examination identified extensive neoplastic cell proliferation with massive necrosis in the tumor tissue. Two types of neoplastic cells were recognized. One type of neoplastic cells were large cells characterized by round to polygonal shape and abundant eosinophilic cytoplasm (referred to as "large cells"). The other neoplastic cells were small, densely proliferated, and had round to irregular shape and scant eosinophilic cytoplasm (referred to as "small cells"). Both types of cells were positive for oligodendrocyte transcription factor 2 and SRY-box transcription factor 10. Glial fibrillary acidic protein was positive in large cells but negative in most small cells. Digital analysis for Ki-67-stained tumor tissues found that total 21.1% ± 6.5% of tumor cells were positive for Ki-67. Based on these findings, we diagnosed malignant oligoastrocytoma in the spinal cord.
Keywords: Ki-67; cat; oligoastrocytoma; spinal cord; tumor.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The authors received no financial support for the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Spinal oligodendroglioma with diffuse arachnoidal dissemination in a Japanese Black heifer.J Vet Med Sci. 1999 Dec;61(12):1323-6. doi: 10.1292/jvms.61.1323. J Vet Med Sci. 1999. PMID: 10651054
-
Feline spinal cord gliomas: Clinicopathologic and diagnostic features of seven cases.J Vet Diagn Invest. 2014 Jul;26(4):513-520. doi: 10.1177/1040638714533118. J Vet Diagn Invest. 2014. PMID: 24821692
-
Oligodendroglioma in the cervical spinal cord of a dog.Vet Pathol. 2004 Sep;41(5):524-6. doi: 10.1354/vp.41-5-524. Vet Pathol. 2004. PMID: 15347828
-
Rosette-Forming Glioneuronal Tumor Originating From the Spinal Cord: Report of 2 Cases and Literature Review.World Neurosurg. 2017 Feb;98:875.e1-875.e7. doi: 10.1016/j.wneu.2016.11.109. Epub 2016 Nov 30. World Neurosurg. 2017. PMID: 27915062 Review.
-
[Glioneuronal tumor with neuropil-like island: report of four cases and review of literature].Zhonghua Bing Li Xue Za Zhi. 2016 May 8;45(5):324-8. doi: 10.3760/cma.j.issn.0529-5807.2016.05.008. Zhonghua Bing Li Xue Za Zhi. 2016. PMID: 27142914 Review. Chinese.
Cited by
-
Feline Facial Spindle Cell Tumors in 29 Cats: Histomorphological and Immunohistochemical Characterization.Animals (Basel). 2024 Apr 4;14(7):1103. doi: 10.3390/ani14071103. Animals (Basel). 2024. PMID: 38612342 Free PMC article.
-
Case report: Surgical treatment of an astrocytoma in the thoracic spinal cord of a cat.Front Vet Sci. 2023 Oct 24;10:1264916. doi: 10.3389/fvets.2023.1264916. eCollection 2023. Front Vet Sci. 2023. PMID: 37941813 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

