Central regulation of body fluid homeostasis
- PMID: 35908954
- PMCID: PMC9363595
- DOI: 10.2183/pjab.98.016
Central regulation of body fluid homeostasis
Abstract
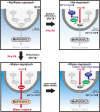
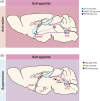
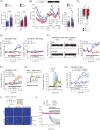
Extracellular fluids, including blood, lymphatic fluid, and cerebrospinal fluid, are collectively called body fluids. The Na+ concentration ([Na+]) in body fluids is maintained at 135-145 mM and is broadly conserved among terrestrial animals. Homeostatic osmoregulation by Na+ is vital for life because severe hyper- or hypotonicity elicits irreversible organ damage and lethal neurological trauma. To achieve "body fluid homeostasis" or "Na homeostasis", the brain continuously monitors [Na+] in body fluids and controls water/salt intake and water/salt excretion by the kidneys. These physiological functions are primarily regulated based on information on [Na+] and relevant circulating hormones, such as angiotensin II, aldosterone, and vasopressin. In this review, we discuss sensing mechanisms for [Na+] and hormones in the brain that control water/salt intake behaviors, together with the responsible sensors (receptors) and relevant neural pathways. We also describe mechanisms in the brain by which [Na+] increases in body fluids activate the sympathetic neural activity leading to hypertension.
Keywords: [Na+] sensor; angiotensin II; blood pressure; body fluid homeostasis; osmosensor; salt appetite; thirst.
Figures
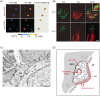



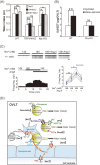
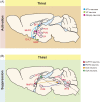

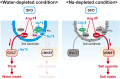



Similar articles
-
Central regulation of body-fluid homeostasis.Trends Neurosci. 2013 Nov;36(11):661-73. doi: 10.1016/j.tins.2013.08.004. Epub 2013 Sep 7. Trends Neurosci. 2013. PMID: 24016361 Review.
-
Hypertension: salt restriction, sodium homeostasis, and other ions.Indian J Med Sci. 2011 Mar;65(3):121-32. Indian J Med Sci. 2011. PMID: 23250294 Review.
-
Brain sodium sensing for regulation of thirst, salt appetite, and blood pressure.Physiol Rep. 2024 Mar;12(5):e15970. doi: 10.14814/phy2.15970. Physiol Rep. 2024. PMID: 38479999 Free PMC article. Review.
-
The neurobiology of thirst and salt appetite.Neuron. 2024 Dec 18;112(24):3999-4016. doi: 10.1016/j.neuron.2024.10.028. Epub 2024 Nov 27. Neuron. 2024. PMID: 39610247 Review.
-
Behavioral responses and fluid regulation in male rats after combined dietary sodium deficiency and water deprivation.Physiol Behav. 2018 Feb 1;184:162-171. doi: 10.1016/j.physbeh.2017.11.025. Epub 2017 Nov 23. Physiol Behav. 2018. PMID: 29174912
Cited by
-
TMEM63B channel is the osmosensor required for thirst drive of interoceptive neurons.Cell Discov. 2024 Jan 3;10(1):1. doi: 10.1038/s41421-023-00628-x. Cell Discov. 2024. PMID: 38172113 Free PMC article.
-
Effect of Short-Term Restraint Stress on the Hypothalamic Transcriptome Profiles of Rats with Inherited Stress-Induced Arterial Hypertension (ISIAH) and Normotensive Wistar Albino Glaxo (WAG) Rats.Int J Mol Sci. 2024 Jun 18;25(12):6680. doi: 10.3390/ijms25126680. Int J Mol Sci. 2024. PMID: 38928385 Free PMC article.
-
The organum vasculosum of the lamina terminalis and subfornical organ: regulation of thirst.Front Neurosci. 2023 Sep 4;17:1223836. doi: 10.3389/fnins.2023.1223836. eCollection 2023. Front Neurosci. 2023. PMID: 37732311 Free PMC article. Review.
-
Effects of Puberty on Blood Pressure Trajectories - Underlying Processes.Curr Hypertens Rep. 2023 Jul;25(7):117-125. doi: 10.1007/s11906-023-01241-9. Epub 2023 Apr 18. Curr Hypertens Rep. 2023. PMID: 37071287 Free PMC article. Review.
-
NaX Channel Is a Physiological [Na+] Detector in Oxytocin- and Vasopressin-Releasing Magnocellular Neurosecretory Cells of the Rat Supraoptic Nucleus.J Neurosci. 2023 Dec 6;43(49):8306-8316. doi: 10.1523/JNEUROSCI.1203-23.2023. J Neurosci. 2023. PMID: 37783507 Free PMC article.
References
-
- Cox P.S., Denton D.A., Mouw D.R., Tarjan E. (1987) Natriuresis induced by localized perfusion within the third cerebral ventricle of sheep. Am. J. Physiol. 252, R1–R6. - PubMed
-
- Park R., Denton D.A., McKinley M.J., Pennington G., Weisinger R.S. (1989) Intracerebroventricular saccharide infusions inhibit thirst induced by systemic hypertonicity. Brain Res. 493, 123–128. - PubMed
-
- McKinley M.J., McAllen R.M., Davern P., Giles M.E., Penschow J., Sunn N., et al. (2003) The sensory circumventricular organs of the mammalian brain. Adv. Anat. Embryol. Cell Biol. 172, III–XII, 1–122, back cover. - PubMed
-
- Johnson A.K., Gross P.M. (1993) Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 7, 678–686. - PubMed

