Bacterial biofilm functionalization through Bap amyloid engineering
- PMID: 35909185
- PMCID: PMC9339546
- DOI: 10.1038/s41522-022-00324-w
Bacterial biofilm functionalization through Bap amyloid engineering
Abstract
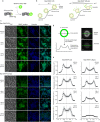
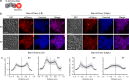
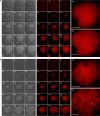
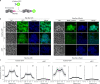
Biofilm engineering has emerged as a controllable way to fabricate living structures with programmable functionalities. The amyloidogenic proteins comprising the biofilms can be engineered to create self-assembling extracellular functionalized surfaces. In this regard, facultative amyloids, which play a dual role in biofilm formation by acting as adhesins in their native conformation and as matrix scaffolds when they polymerize into amyloid-like fibrillar structures, are interesting candidates. Here, we report the use of the facultative amyloid-like Bap protein of Staphylococcus aureus as a tool to decorate the extracellular biofilm matrix or the bacterial cell surface with a battery of functional domains or proteins. We demonstrate that the localization of the functional tags can be change by simply modulating the pH of the medium. Using Bap features, we build a tool for trapping and covalent immobilizing molecules at bacterial cell surface or at the biofilm matrix based on the SpyTag/SpyCatcher system. Finally, we show that the cell wall of several Gram-positive bacteria could be functionalized through the external addition of the recombinant engineered Bap-amyloid domain. Overall, this work shows a simple and modulable system for biofilm functionalization based on the facultative protein Bap.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Staphylococcal Bap Proteins Build Amyloid Scaffold Biofilm Matrices in Response to Environmental Signals.PLoS Pathog. 2016 Jun 21;12(6):e1005711. doi: 10.1371/journal.ppat.1005711. eCollection 2016 Jun. PLoS Pathog. 2016. PMID: 27327765 Free PMC article.
-
Antibiofilm activity of flavonoids on staphylococcal biofilms through targeting BAP amyloids.Sci Rep. 2020 Nov 3;10(1):18968. doi: 10.1038/s41598-020-75929-2. Sci Rep. 2020. PMID: 33144670 Free PMC article.
-
Bap: A New Type of Functional Amyloid.Trends Microbiol. 2016 Sep;24(9):682-684. doi: 10.1016/j.tim.2016.07.004. Epub 2016 Jul 21. Trends Microbiol. 2016. PMID: 27451288
-
Protein-based biofilm matrices in Staphylococci.Front Cell Infect Microbiol. 2014 Dec 10;4:171. doi: 10.3389/fcimb.2014.00171. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25540773 Free PMC article. Review.
-
Functional Amyloid and Other Protein Fibers in the Biofilm Matrix.J Mol Biol. 2018 Oct 12;430(20):3642-3656. doi: 10.1016/j.jmb.2018.07.026. Epub 2018 Aug 8. J Mol Biol. 2018. PMID: 30098341 Free PMC article. Review.
Cited by
-
Hierarchical organization and assembly of the archaeal cell sheath from an amyloid-like protein.Nat Commun. 2023 Oct 23;14(1):6720. doi: 10.1038/s41467-023-42368-2. Nat Commun. 2023. PMID: 37872154 Free PMC article.
-
Biofilm-Associated Amyloid Proteins Linked with the Progression of Neurodegenerative Diseases.Int J Mol Sci. 2025 Mar 17;26(6):2695. doi: 10.3390/ijms26062695. Int J Mol Sci. 2025. PMID: 40141340 Free PMC article. Review.
-
Amyloid Fibrils Produced by Streptococcus sanguinis Contribute to Biofilm Formation and Immune Evasion.Int J Mol Sci. 2023 Oct 28;24(21):15686. doi: 10.3390/ijms242115686. Int J Mol Sci. 2023. PMID: 37958670 Free PMC article.
-
Light-activated nanoclusters with tunable ROS for wound infection treatment.Bioact Mater. 2024 Jul 30;41:385-399. doi: 10.1016/j.bioactmat.2024.07.009. eCollection 2024 Nov. Bioact Mater. 2024. PMID: 39184828 Free PMC article.
-
Strategies and materials for the prevention and treatment of biofilms.Mater Today Bio. 2023 Oct 2;23:100827. doi: 10.1016/j.mtbio.2023.100827. eCollection 2023 Dec. Mater Today Bio. 2023. PMID: 37859998 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

