Neurotrophin Crosstalk in the Etiology and Treatment of Neuropsychiatric and Neurodegenerative Disease
- PMID: 35909451
- PMCID: PMC9335126
- DOI: 10.3389/fnmol.2022.932497
Neurotrophin Crosstalk in the Etiology and Treatment of Neuropsychiatric and Neurodegenerative Disease
Abstract
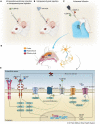
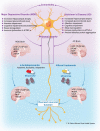
This article reviews the current progress in our understanding of the mechanisms by which growth factors, including brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF), and select neurotrophin-regulated gene products, such as VGF (non-acronymic) and VGF-derived neuropeptides, function in the central nervous system (CNS) to modulate neuropsychiatric and neurodegenerative disorders, with a discussion of the possible therapeutic applications of these growth factors to major depressive disorder (MDD) and Alzheimer's disease (AD). BDNF and VEGF levels are generally decreased regionally in the brains of MDD subjects and in preclinical animal models of depression, changes that are associated with neuronal atrophy and reduced neurogenesis, and are reversed by conventional monoaminergic and novel ketamine-like antidepressants. Downstream of neurotrophins and their receptors, VGF was identified as a nerve growth factor (NGF)- and BDNF-inducible secreted protein and neuropeptide precursor that is produced and trafficked throughout the CNS, where its expression is greatly influenced by neuronal activity and exercise, and where several VGF-derived peptides modulate neuronal activity, function, proliferation, differentiation, and survival. Moreover, levels of VGF are reduced in the CSF of AD subjects, where it has been repetitively identified as a disease biomarker, and in the hippocampi of subjects with MDD, suggesting possible shared mechanisms by which reduced levels of VGF and other proteins that are similarly regulated by neurotrophin signaling pathways contribute to and potentially drive the pathogenesis and progression of co-morbid neuropsychiatric and neurodegenerative disorders, particularly MDD and AD, opening possible therapeutic windows.
Keywords: ADNP (activity dependent neuroprotective protein); Alzheimer’s disease; BDNF (brain derived neurotrophic factor); MDD (major depressive disorder); TLQP-62; TrkB; VEGF – vascular endothelial growth factor; VGF.
Copyright © 2022 Joshi and Salton.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Asano T., Koizumi S., Takagi A., Hatori T., Kuwabara K., Fujino O., et al. (2011). Identification of a novel biomarker candidate, a 4.8-kDa peptide fragment from a neurosecretory protein VGF precursor, by proteomic analysis of cerebrospinal fluid from children with acute encephalopathy using SELDI-TOF-MS. BMC Neurol. 11:101. 10.1186/1471-2377-11-101 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

