Gram-negative bacteria act as a reservoir for aminoglycoside antibiotics that interact with host factors to enhance bacterial killing in a mouse model of pneumonia
- PMID: 35909464
- PMCID: PMC9326624
- DOI: 10.1093/femsmc/xtac016
Gram-negative bacteria act as a reservoir for aminoglycoside antibiotics that interact with host factors to enhance bacterial killing in a mouse model of pneumonia
Abstract
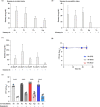
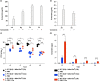
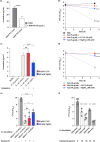
In vitro exposure of multiple Gram-negative bacteria to an aminoglycoside (AG) antibiotic has previously been demonstrated to result in bacterial alterations that interact with host factors to suppress Gram-negative pneumonia. However, the mechanisms resulting in suppression are not known. Here, the hypothesis that Gram-negative bacteria bind and retain AGs, which are introduced into the lung and interact with host defenses to affect bacterial killing, was tested. Following in vitro exposure of one of several, pathogenic Gram-negative bacteria to the AG antibiotics kanamycin or gentamicin, AGs were detected in bacterial cell pellets (up to 208 μg/mL). Using inhibitors of AG binding and internalization, the bacterial outer membrane was implicated as the predominant kanamycin and gentamicin reservoir. Following intranasal administration of gentamicin-bound bacteria or gentamicin solution at the time of infection with live, AG-naïve bacteria, gentamicin was detected in the lungs of infected mice (up to 8 μg/g). Co-inoculation with gentamicin-bound bacteria resulted in killing of AG-naïve bacteria by up to 3-log10, mirroring the effects of intranasal gentamicin treatment. In vitro killing of AG-naïve bacteria mediated by kanamycin-bound bacteria required the presence of detergents or pulmonary surfactant, suggesting that increased bacterial killing inside the murine lung is facilitated by the detergent component of pulmonary surfactant. These findings demonstrate that Gram-negative bacteria bind and retain AGs that can interact with host-derived pulmonary surfactant to enhance bacterial killing in the lung. This may help explain why AGs appear to have unique efficacy in the lung and might expand their clinical utility.
Keywords: Gram-negative; aminoglycosides; antibiotics; bacterial pneumonia; host–microbe interactions; pulmonary surfactant.
© The Author(s) 2022. Published by Oxford University Press on behalf of FEMS.
Figures


Similar articles
-
A novel ruthenium-silver based antimicrobial potentiates aminoglycoside activity against Pseudomonas aeruginosa.mSphere. 2023 Oct 24;8(5):e0019023. doi: 10.1128/msphere.00190-23. Epub 2023 Aug 30. mSphere. 2023. PMID: 37646510 Free PMC article.
-
Antibiotic resistance patterns during aminoglycoside restriction.Am J Med Sci. 1985 Dec;290(6):223-7. doi: 10.1097/00000441-198512000-00001. Am J Med Sci. 1985. PMID: 3936358 Clinical Trial.
-
Erratum: High-Throughput Identification of Resistance to Pseudomonas syringae pv. Tomato in Tomato using Seedling Flood Assay.J Vis Exp. 2023 Oct 18;(200). doi: 10.3791/6576. J Vis Exp. 2023. PMID: 37851522
-
[Biochemical and genetic mechanisms for bacteria to acquire aminoglycoside antibiotic resistance].Nihon Rinsho. 1997 May;55(5):1231-7. Nihon Rinsho. 1997. PMID: 9155180 Review. Japanese.
-
Parallel pathways in the biosynthesis of aminoglycoside antibiotics.F1000Res. 2017 May 18;6:F1000 Faculty Rev-723. doi: 10.12688/f1000research.11104.1. eCollection 2017. F1000Res. 2017. PMID: 28620453 Free PMC article. Review.
References
-
- Anderson MS, Robertson AD, Macher Iet al. . Biosynthesis of lipid a in escherichiacoli: identification of UDP-3-O-[(R)-3-hydroxymyristoyl]-alpha-D-glucosamine as a precursor of UDP-N2,O3-bis[(R)-3-hydroxymyristoyl]-alpha-D-glucosamine. Biochemistry. 1988;27:1908–17. - PubMed
-
- Boselli E, Breilh D, Djabarouti Set al. . Reliability of mini-bronchoalveolar lavage for the measurement of epithelial lining fluid concentrations of tobramycin in critically ill patients. Intensive Care Med. 2007;33:1519–23. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
