One-Step Homogeneous Immunoassay for the Detection of Influenza Virus Using Switching Peptide and Graphene Quencher
- PMID: 35909466
- PMCID: PMC9326414
- DOI: 10.1007/s13206-022-00076-x
One-Step Homogeneous Immunoassay for the Detection of Influenza Virus Using Switching Peptide and Graphene Quencher
Abstract
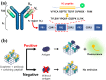
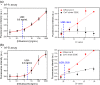
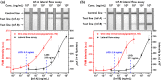
One-step homogeneous immunoassay was developed for detecting influenza viruses A and B (Inf-A and Inf-B) using the switching peptide H2. As the fluorescence-labeled switching peptide dissociated from the binding pocket of detection antibodies, the fluorescence signal could be directly generated by the binding of Inf-A and Inf-B without washing (i.e., one-step immunoassay). For the one-step homogeneous immunoassay with detection antibodies in solution, graphene was labeled with the antibodies as a fluorescence quencher. To test the feasibility of the homogeneous one-step immunoassay, the stability of the antibody complex with the switching peptide was evaluated under different pH and salt conditions. The one-step homogeneous immunoassay with switching peptide was conducted using influenza virus antigens in phosphate-buffered saline and real samples with inactivated Inf-A and Inf-B spiked in serum. Finally, the one-step homogeneous immunoassay results were compared with those of commercially available lateral flow immunoassays.
Keywords: Influenza-A and influenza-B virus; Lateral flow immunoassay; One-step homogeneous immunoassay; Switching peptide.
© The Korean BioChip Society 2022.
Conflict of interest statement
Conflict of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Makabe K, Nakanishi T, Tsumoto K, Tanaka Y, Kondo H, Umetsu M, Sone Y, Asano R, Kumagai I. Thermodynamic consequences of mutations in vernier zone residues of a humanized anti-human epidermal growth factor receptor murine antibody, 528. J. Biol. Chem. 2008;283:1156–1166. doi: 10.1074/jbc.M706190200. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
