Anti-Metalloprotease P-I Single-Domain Antibodies: Tools for Next-Generation Snakebite Antivenoms
- PMID: 35909472
- PMCID: PMC9325618
- DOI: 10.1155/2022/2748962
Anti-Metalloprotease P-I Single-Domain Antibodies: Tools for Next-Generation Snakebite Antivenoms
Abstract
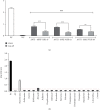
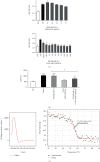
In order to address the global antivenom crisis, novel antivenoms need to present high therapeutic efficacy, broad neutralization ability against systemic and local damage, sufficient safety, and cost-effectiveness. Due to biological characteristics of camelid single-domain antibodies (VHH) such as high affinity, their ability to penetrate dense tissues, and facility for genetic manipulation, their application in antivenoms has expanded considerably. VHHs that are active against the metalloprotease BjussuMP-II from the snake Bothrops jararacussu were selected. After isolation of BjussuMP-II, a camelid was immunized with the purified toxin in order to construct the recombinant phage library. Following a round of biopanning, 52% of the selected clones were able to recognize BjussuMP-II in an ELISA assay. After sequencing, seven sequence profiles were identified. One selected clone (VHH61) showed cross-reactivity to B. brazili venom, but did not recognize the Crotalus and Lachesis genera, indicating specificity for the Bothrops genus. Through in vitro tests, the capacity to neutralize the toxicity triggered by BjussuMP-II was observed. Circular dichroism spectroscopy indicated a robust secondary structure for VHH61, and the calculated melting temperature (T M) for the clone was 56.4°C. In silico analysis, through molecular docking of anti-BjussuMP-II VHHs with metalloprotease, revealed their potential interaction with amino acids present in regions critical for the toxin's conformation and stability. The findings suggest that anti-BjussuMP-II VHHs may be beneficial in the development of next-generation antivenoms.
Copyright © 2022 Marcela C. S. Silva et al.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Inhibition of the Myotoxicity Induced by Bothrops jararacussu Venom and Isolated Phospholipases A2 by Specific Camelid Single-Domain Antibody Fragments.PLoS One. 2016 Mar 30;11(3):e0151363. doi: 10.1371/journal.pone.0151363. eCollection 2016. PLoS One. 2016. PMID: 27028872 Free PMC article.
-
Preclinical evaluation of single domain antibody efficacy in mitigating local tissue damage induced by Bothrops snake envenomation.Int Immunopharmacol. 2024 Jun 15;134:112215. doi: 10.1016/j.intimp.2024.112215. Epub 2024 May 13. Int Immunopharmacol. 2024. PMID: 38744173
-
Antivenomics and in vivo preclinical efficacy of six Latin American antivenoms towards south-western Colombian Bothrops asper lineage venoms.PLoS Negl Trop Dis. 2021 Feb 1;15(2):e0009073. doi: 10.1371/journal.pntd.0009073. eCollection 2021 Feb. PLoS Negl Trop Dis. 2021. PMID: 33524033 Free PMC article.
-
Engineering of single-domain antibodies for next-generation snakebite antivenoms.Int J Biol Macromol. 2021 Aug 31;185:240-250. doi: 10.1016/j.ijbiomac.2021.06.043. Epub 2021 Jun 9. Int J Biol Macromol. 2021. PMID: 34118288 Review.
-
Immunological profile of antivenoms: preclinical analysis of the efficacy of a polyspecific antivenom through antivenomics and neutralization assays.J Proteomics. 2014 Jun 13;105:340-50. doi: 10.1016/j.jprot.2014.02.021. Epub 2014 Feb 28. J Proteomics. 2014. PMID: 24583507 Review.
Cited by
-
Revolutionizing snakebite care with novel antivenoms: Breakthroughs and barriers.Heliyon. 2024 Jan 30;10(3):e25531. doi: 10.1016/j.heliyon.2024.e25531. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38333815 Free PMC article. Review.
-
Targets Involved in the Pharmacology of Bothrops Snakebite: Statu Quo and Future Perspectives.Curr Drug Targets. 2025;26(7):454-469. doi: 10.2174/0113894501352925250225045555. Curr Drug Targets. 2025. PMID: 40017248 Review.
-
Biological and Medical Aspects Related to South American Rattlesnake Crotalus durissus (Linnaeus, 1758): A View from Colombia.Toxins (Basel). 2022 Dec 15;14(12):875. doi: 10.3390/toxins14120875. Toxins (Basel). 2022. PMID: 36548772 Free PMC article. Review.
References
-
- World Health Organization Snake Antivenoms. Envenenamento por Picada de Cobra . 2021. http://www.who.int/es/news-room/fact-sheets/detail/snakebite-envenoming .
-
- Resiere D., Monteiro W., Houcke S., et al. Bothrops snakebite envenomings in the Amazon Region. Current Tropical Medicine Reports . 2020;7:48–60. doi: 10.1007/s40475-020-00203-4. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

