Role of Acetaldehyde in Ethanol Reversal of Tolerance to Morphine-Induced Respiratory Depression in Mice
- PMID: 35909497
- PMCID: PMC7613180
- DOI: 10.3389/adar.2021.10143
Role of Acetaldehyde in Ethanol Reversal of Tolerance to Morphine-Induced Respiratory Depression in Mice
Abstract
Background: Opioid users regularly consume other drugs such as alcohol (ethanol). Acute administration of ethanol rapidly reverses tolerance to morphine-induced respiratory depression. However, recent research has suggested that the primary metabolite of ethanol, acetaldehyde, may play a key role in mediating the CNS effects seen after ethanol consumption. This research investigated the role of acetaldehyde in ethanol reversal of tolerance to morphine-induced respiratory depression.
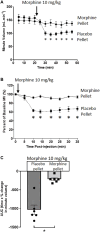
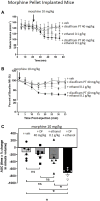
Methods: Tolerance was induced in mice by 6-days implantation of a 75 mg morphine pellet with control mice implanted with a placebo pellet. Tolerance was assessed by acute morphine administration on day 6 and respiration measured by plethysmography. Levels of acetaldehyde were inhibited or enhanced by pre-treatments with the acetaldehyde chelator D-penicillamine and the inhibitor of acetaldehyde dehydrogenase disulfiram respectively.
Results: Morphine pellet implanted mice displayed tolerance to an acute dose of morphine compared to placebo pellet implanted controls. Acute acetaldehyde administration dose-dependently reversed tolerance to morphine respiratory depression. As previously demonstrated, ethanol reversed morphine tolerance, and this was inhibited by D-penicillamine pre-treatment. An acute, low dose of ethanol that did not significantly reverse morphine tolerance was able to do so following disulfiram pre-treatment.
Conclusion: These data suggest that acetaldehyde, the primary metabolite of ethanol, is responsible for the reversal of morphine tolerance observed following ethanol administration.
Keywords: acetaldehyde; ethanol; morphine; opioid; polydrug; polypharmacology; respiration; tolerance.
Conflict of interest statement
Conflict of Interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

