PEGylated Recombinant Human Growth Hormone Jintrolong® Exhibits Good Long-Term Safety in Cynomolgus Monkeys and Human Pediatric Growth Hormone Deficiency Patients
- PMID: 35909512
- PMCID: PMC9336684
- DOI: 10.3389/fendo.2022.821588
PEGylated Recombinant Human Growth Hormone Jintrolong® Exhibits Good Long-Term Safety in Cynomolgus Monkeys and Human Pediatric Growth Hormone Deficiency Patients
Abstract
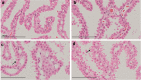
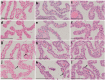
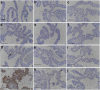
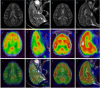
Jintrolong® is a long-acting PEGylated recombinant human growth hormone (PEG-rhGH) developed for weekly injection in patients with pediatric growth hormone deficiency (PGHD). Although PEG modification of therapeutic proteins is generally considered safe, concerns persist about the potential for adverse vacuolation in tissues with long-term exposure to PEG-included therapies, particularly in children. We assessed the safety of Jintrolong® in cynomolgus monkeys with an examination of vacuolation in the brain choroid plexus (CP) and reported long-term clinical safety data obtained from children with PGHD. The toxicity of Jintrolong® was assessed following the 52-week administration with doses at 0.3, 1, or 3 mg/kg/week. The levels of vacuolation of CP in animals were dose-dependent and at least partially reversible after a 104- or 157-week recovery period. Vacuolation in the CP epithelium did not lead to obvious subcellular structural or cell functional abnormalities. Compared with the clinical dose of 0.2 mg/kg/week Jintrolong® in PGHD patients, exposure in monkeys under NOAEL 3 mg/kg/week exhibited safety margins greater than 120.5, the predicted minimum dose to induce vacuolation in monkeys is equivalent to 1.29 mg/kg/week in humans, which is 6.45-fold higher than the clinical dose. The safety data acquired in clinical trials for Jintrolong® were also analyzed, which included phase III (360 patients), phase IV (3,000 patients) of 26-week treatment, and a follow-up study with treatment lasting for 3 years. There was no statistically significant difference in the incidence of adverse reactions between the Jintrolong® group and the daily rhGH control group (no PEG), and no new adverse effects (AE) were observed in the Jintrolong® group at the clinical therapeutic dose of 0.2 mg/kg/week.
Keywords: AE; CNS; GHD; MRI; PEG-rhGH; TEM; choroid plexus (CP); vacuolation.
Copyright © 2022 Wu, Zhou, Wu, Zhou, Li, Zhang, Zuo, Yin, Hou, Wang, Gao, Luo, Jin, Zhong, Wang and Luo.
Conflict of interest statement
JZ, QZ, XLi, TL, LJ and EZ are employees of GeneScience Pharmaceuticals Co., Ltd. CW, CZ, YZ and JY are employees by JOINN Laboratories Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Long-acting PEGylated recombinant human growth hormone (Jintrolong) for children with growth hormone deficiency: phase II and phase III multicenter, randomized studies.Eur J Endocrinol. 2017 Aug;177(2):195-205. doi: 10.1530/EJE-16-0905. Epub 2017 May 31. Eur J Endocrinol. 2017. PMID: 28566441 Free PMC article. Clinical Trial.
-
Comparative pharmacokinetics and pharmacodynamics of a PEGylated recombinant human growth hormone and daily recombinant human growth hormone in growth hormone-deficient children.Drug Des Devel Ther. 2015 Dec 18;10:13-21. doi: 10.2147/DDDT.S93183. eCollection 2016. Drug Des Devel Ther. 2015. PMID: 26719670 Free PMC article. Clinical Trial.
-
A long-acting pegylated recombinant human growth hormone (Jintrolong® ) in healthy adult subjects: Two single-dose trials evaluating safety, tolerability and pharmacokinetics.J Clin Pharm Ther. 2018 Oct;43(5):640-646. doi: 10.1111/jcpt.12732. Epub 2018 Jun 29. J Clin Pharm Ther. 2018. PMID: 29959799 Clinical Trial.
-
Safety of long-term use of daily and long-acting growth hormone in growth hormone-deficient adults on cancer risk.Best Pract Res Clin Endocrinol Metab. 2023 Dec;37(6):101817. doi: 10.1016/j.beem.2023.101817. Epub 2023 Aug 24. Best Pract Res Clin Endocrinol Metab. 2023. PMID: 37643936 Review.
-
Long-Acting Growth Hormone Preparations and Their Use in Children with Growth Hormone Deficiency.Horm Res Paediatr. 2023;96(6):553-559. doi: 10.1159/000523791. Epub 2022 Feb 25. Horm Res Paediatr. 2023. PMID: 35220308 Review.
Cited by
-
Growth hormone receptor agonists and antagonists: From protein expression and purification to long-acting formulations.Protein Sci. 2023 Sep;32(9):e4727. doi: 10.1002/pro.4727. Protein Sci. 2023. PMID: 37428391 Free PMC article. Review.
-
Optimizing Pharmacological and Immunological Properties of Therapeutic Proteins Through PEGylation: Investigating Key Parameters and Their Impact.Drug Des Devel Ther. 2024 Nov 7;18:5041-5062. doi: 10.2147/DDDT.S481420. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39529843 Free PMC article. Review.
-
Comparison between long-acting pegylated and daily recombinant human growth hormone for pediatric growth hormone deficiency a systematic review.Sci Rep. 2025 Jul 23;15(1):26746. doi: 10.1038/s41598-025-10613-x. Sci Rep. 2025. PMID: 40702077 Free PMC article.
-
Long-Acting Growth Hormone Therapy in Pediatric Growth Hormone Deficiency: A Consensus Statement.J Clin Endocrinol Metab. 2025 Mar 17;110(4):e1232-e1240. doi: 10.1210/clinem/dgae834. J Clin Endocrinol Metab. 2025. PMID: 39672599 Free PMC article.
-
Long-acting growth hormone in the treatment of growth hormone deficiency in children: a systematic literature review and network meta-analysis.Sci Rep. 2024 Apr 5;14(1):8061. doi: 10.1038/s41598-024-58616-4. Sci Rep. 2024. PMID: 38580693 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

