Transcriptome Analysis Reveals the Mechanism of Natural Ovarian Ageing
- PMID: 35909541
- PMCID: PMC9329525
- DOI: 10.3389/fendo.2022.918212
Transcriptome Analysis Reveals the Mechanism of Natural Ovarian Ageing
Abstract
Background: The decline in the quantity and quality of oocytes due to ovarian ageing in women is now a significant threat to reproductive health today as the concept of delayed fertility becomes widespread. However, the molecular mechanisms of natural ovarian ageing have not been fully elucidated.
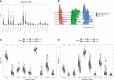
Method: Here, we used transcriptomic data from 180 normal ovarian tissues from GTEx V8 to analyze the expression profile of ovarian tissues from women with age segments of 20-29 (22 individuals), 30-39 (14 individuals), 40-49 (37 individuals), 50-59 (61 individuals), 60-69 (42 individuals), and 70-79 (4 individuals), respectively. XCELL was used to assess the infiltration score of 64 cell types of the ovary. WGCNA was used to characterize the co-expression network during the natural aging of the ovary. ClusterprofileR was used for functional enrichment analysis of co-expression modules. MsViper was used for master regulator analysis.
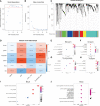
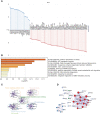
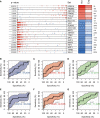
Results: The infiltration score of endothelial cells and activated antigen-presenting cells during natural ovarian ageing increased significantly at ages 30-39, 40-49, and then decreased, whereas CD4+ Tcm increased with age. WGCNA identified six co-expression modules from ovarian tissue transcriptomic data species. The red module was significantly and positively correlated with senescence and CD4+ Tcm, and the turquoise module was significantly and positively correlated with Endothelial Cells. We further explored ovarian tissue for women aged 20-29 and 30-39 years. The GSEA results showed that the Chemokine signaling pathway was significantly activated in the 30-39-year-old group, while Oocyte meiosis was significantly inhibited. Finally, the results of msviper found that transcription factors such as KDM1A, PRDM5, ZNF726, PPARG, FOXJ2, and GLI2 were mainly activated in the 20-29 years group, while VAV1, RUNX3, ZC3H12D, MYCL, and IRF5 were mainly activated in the 30-39 years group and that these transcription factor activities were diagnostic of natural ovarian ageing (AUC: 0.65-0.71).
Conclusion: Natural ageing of the ovary is significantly correlated with immune cell infiltration and activation of inflammation-related signaling pathways, with inflammation levels reaching a maximum during early ovarian ageing (30-39, 40-49) and then gradually decreasing after that. These studies provide a research basis for exploring the mechanisms of natural ovarian ageing.
Keywords: aging; inflammation; mechanism; ovary; reproduction.
Copyright © 2022 Chen, Ding, Wu, Qiu, Li, Ye and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Wang S, Zheng Y, Li J, Yu Y, Zhang W, Song M, et al. Single-Cell Transcriptomic Atlas of Primate Ovarian Aging. (2020) 180:585–600.e19. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

