Focusing on OB-OC-MΦ Axis and miR-23a to Explore the Pathogenesis and Treatment Strategy of Osteoporosis
- PMID: 35909545
- PMCID: PMC9329542
- DOI: 10.3389/fendo.2022.891313
Focusing on OB-OC-MΦ Axis and miR-23a to Explore the Pathogenesis and Treatment Strategy of Osteoporosis
Abstract
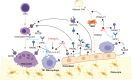
Osteoporosis is a bone metabolic disorder characterized by decreased bone density and deteriorated microstructure, which increases the risk of fractures. The imbalance between bone formation and bone resorption results in the occurrence and progression of osteoporosis. Osteoblast-mediated bone formation, osteoclast-mediated bone resorption and macrophage-regulated inflammatory response play a central role in the process of bone remodeling, which together maintain the balance of the osteoblast-osteoclast-macrophage (OB-OC-MΦ) axis under physiological conditions. Bone formation and bone resorption disorders caused by the imbalance of OB-OC-MΦ axis contribute to osteoporosis. Many microRNAs are involved in the regulation of OB-OC-MΦ axis homeostasis, with microRNA-23a (miR-23a) being particularly crucial. MiR-23a is highly expressed in the pathological process of osteoporosis, which eventually leads to the occurrence and further progression of osteoporosis by inhibiting osteogenesis, promoting bone resorption and inflammatory polarization of macrophages. This review focuses on the role and mechanism of miR-23a in regulating the OB-OC-MΦ axis to provide new clinical strategies for the prevention and treatment of osteoporosis.
Keywords: cytokine; macrophage; miR-23a; osteoblast (OB); osteoclast (OC); osteoporosis.
Copyright © 2022 Ma, Zhu, Ke, Chen, Hu and Xie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

