Therapy With Intravenous Methylprednisolone Pulses Is Associated With Loss of Bone Microarchitecture in Trabecular Bone Score -Assessment Among Patients With Moderate-to-Severe Graves' Orbitopathy: A Pilot Study
- PMID: 35909547
- PMCID: PMC9331277
- DOI: 10.3389/fendo.2022.893600
Therapy With Intravenous Methylprednisolone Pulses Is Associated With Loss of Bone Microarchitecture in Trabecular Bone Score -Assessment Among Patients With Moderate-to-Severe Graves' Orbitopathy: A Pilot Study
Abstract
Background: Therapy with intravenous glucocorticoids (GCs) is associated with various side effects, however, the impact on bone remains elusive. Trabecular bone score (TBS) is a diagnostic tool providing information on bone microarchitecture based on images obtained from dual-energy X-ray absorptiometry. We investigated the influence of the intravenous methylprednisolone (IVMP) pulse administration on TBS in patients with moderate-to-severe Graves' orbitopathy (GO).
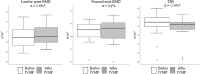
Methods: Fifteen patients with GO were treated with 12 IVMP pulses (6x0.5g, 6x0.25 g on a weekly schedule). They received supplementation with 2000 IU of vitamin D and 1.0 g of calcium throughout the study period. TBS was assessed at baseline and after last IVMP pulse. To determine the difference between values at baseline and after treatment the least significant change (LSC) methodology was used. We compared pre- and posttreatment mean TBS values.
Results: We found a significant decrease of TBS in 5 out of 15 (33%) patients. Mean TBS value decreased becoming 2.4% lower than at baseline (p<0.05).
Conclusions: IVMP pulse therapy exerts negative effect on bone microarchitecture in TBS assessment. The analysis of the clinical risk factors for osteoporosis and the evaluation of bone mineral density and TBS should be considered before initiating IVMP therapy.
Keywords: EUGOGO; graves’ ophthalmopathy; graves’ orbitopathy; methylprednisolone; trabecular bone score.
Copyright © 2022 Rymuza, Pelewicz, Przedlacki and Miśkiewicz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Pelewicz K, Szewczyk S, Miśkiewicz P. Treatment With Intravenous Methylprednisolone in Patients With Graves' Orbitopathy Significantly Affects Adrenal Function: Assessment of Serum, Salivary Cortisol and Serum Dehydroepiandrosterone Sulfate. J Clin Med (2020) 10:3233. doi: 10.3390/jcm9103233 - DOI - PMC - PubMed
-
- Marcocci C, Watt T, Altea MA, Rasmussen AK, Feldt-Rasmussen U, Orgiazzi J, et al. Fatal and non-Fatal Adverse Events of Glucocorticoid Therapy for Graves' Orbitopathy: A Questionnaire Survey Among Members of the European Thyroid Association. Eur. J Endocrinol (2012) 166:247–53. doi: 10.1530/EJE-11-0779 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

