Spermatozoa Develop Molecular Machinery to Recover From Acute Stress
- PMID: 35909555
- PMCID: PMC9329690
- DOI: 10.3389/fendo.2022.896193
Spermatozoa Develop Molecular Machinery to Recover From Acute Stress
Abstract
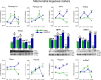
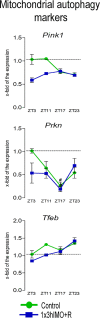
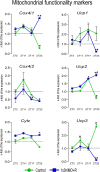
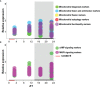
This study was designed to search for the possible mechanism(s) of male (in/sub)fertility by following the molecular response of spermatozoa on acute psychological stress (the most common stress in human society) and on a 20-h time-dependent recovery period. To mimic in vivo acute stress, the rats were exposed to immobilization once every 3 h. The recovery periods were as follows: 0 (immediately after stress and 3 h after the light is on-ZT3), 8 (ZT11), 14 (ZT17), and 20 (ZT23) h after stress. Results showed that acute stress provoked effects evident 20 h after the end of the stress period. Numbers of spermatozoa declined at ZT17 and ZT23, while functionality decreased at ZT3 and ZT11, but recovered at ZT17 and ZT23. Transcriptional profiles of 91% (20/22) of tracked mitochondrial dynamics and functionality markers and 91% (20/22) of signaling molecules regulating both mitochondrial dynamics and spermatozoa number/functionality were disturbed after acute stress and during the recovery period. Most of the changes presented as increased transcription or protein expression at ZT23. The results of the principal component analysis (PCA) showed the clear separation of acute stress recovery effects during active/dark and inactive/light phases. The physiological relevance of these results is the recovered positive-acrosome-reaction, suggesting that molecular events are an adaptive mechanism, regulated by acute stress response signaling. The results of the PCA confirmed the separation of the effects of acute stress recovery on gene expression related to mitochondrial dynamics, cAMP, and MAPK signaling. The transcriptional patterns were different during the active and inactive phases. Most of the transcripts were highly expressed during the active phase, which is expected given that stress occurred at the beginning of the inactive phase. To the best of our knowledge, our results provide a completely new view and the first presentation of the markers of mitochondrial dynamics network in spermatozoa and their correlation with signaling molecules regulating both mitochondrial dynamics and spermatozoa number and functionality during recovery from acute stress. Moreover, the interactions between the proteins important for spermatozoa homeostasis and functionality (MFN2 and PRKA catalytic subunit, MFN2 and p38MAPK) are shown for the first time. Since the existing literature suggests the importance of semen quality and male fertility not only as the fundamental marker of reproductive health but also as the fundamental biomarkers of overall health and harbingers for the development of comorbidity and mortality, we anticipate our result to be a starting point for more investigations considering the mitochondrial dynamics markers or their transcriptional profiles as possible predictors of (in/sub)fertility.
Keywords: MAPK signaling markers; acute psychological stress; cAMP signaling markers; mitochondrial dynamics and functionality markers; spermatozoa number and functionality; stress recovery.
Copyright © 2022 Starovlah, Radovic Pletikosic, Tomanic, Medar, Kostic and Andric.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

