The Role of Epigenetic Change in Therapy-Induced Neuroendocrine Prostate Cancer Lineage Plasticity
- PMID: 35909568
- PMCID: PMC9329809
- DOI: 10.3389/fendo.2022.926585
The Role of Epigenetic Change in Therapy-Induced Neuroendocrine Prostate Cancer Lineage Plasticity
Abstract
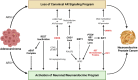
The androgen receptor (AR) signaling pathway is critical for growth and differentiation of prostate cancer cells. For that reason, androgen deprivation therapy with medical or surgical castration is the principal treatment for metastatic prostate cancer. More recently, new potent AR signaling inhibitors (ARSIs) have been developed. These drugs improve survival for men with metastatic castration-resistant prostate cancer (CRPC), the lethal form of the disease. However, ARSI resistance is nearly universal. One recently appreciated resistance mechanism is lineage plasticity or switch from an AR-driven, luminal differentiation program to an alternate differentiation program. Importantly, lineage plasticity appears to be increasing in incidence in the era of new ARSIs, strongly implicating AR suppression in this process. Lineage plasticity and shift from AR-driven tumors occur on a continuum, ranging from AR-expressing tumors with low AR activity to AR-null tumors that have activation of alternate differentiation programs versus the canonical luminal program found in AR-driven tumors. In many cases, AR loss coincides with the activation of a neuronal program, most commonly exemplified as therapy-induced neuroendocrine prostate cancer (t-NEPC). While genetic events clearly contribute to prostate cancer lineage plasticity, it is also clear that epigenetic events-including chromatin modifications and DNA methylation-play a major role. Many epigenetic factors are now targetable with drugs, establishing the importance of clarifying critical epigenetic factors that promote lineage plasticity. Furthermore, epigenetic marks are readily measurable, demonstrating the importance of clarifying which measurements will help to identify tumors that have undergone or are at risk of undergoing lineage plasticity. In this review, we discuss the role of AR pathway loss and activation of a neuronal differentiation program as key contributors to t-NEPC lineage plasticity. We also discuss new epigenetic therapeutic strategies to reverse lineage plasticity, including those that have recently entered clinical trials.
Keywords: Androgen Receptor (AR); Lineage plasticity; Neuroendocrine prostate cancer (NEPC); epigenetics; transdifferentiation.
Copyright © 2022 Storck, May, Westbrook, Duan, Morrissey, Yates and Alumkal.
Conflict of interest statement
JJA reports consulting and speaker’s fees from Astellas Pharma, consulting fees from Dendreon, consulting fees from Merck, consulting fees from Bristol Myers Squibb, and research support to his institution from Astellas Pharma, Zenith Epigenetics, and Beactica. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

