Cell death induction in Aspergillus fumigatus: accentuating drug toxicity through inhibition of the unfolded protein response (UPR)
- PMID: 35909601
- PMCID: PMC9325865
- DOI: 10.1016/j.crmicr.2022.100119
Cell death induction in Aspergillus fumigatus: accentuating drug toxicity through inhibition of the unfolded protein response (UPR)
Abstract
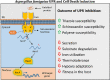
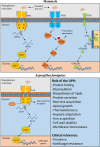
One of the most potent opportunistic fungal pathogens of humans is Aspergillus fumigatus, an environmental mold that causes a life-threatening pneumonia with a high rate of morbidity and mortality. Despite advances in therapy, issues of drug toxicity and antifungal resistance remain an obstacle to effective therapy. This underscores the need for more information on fungal pathways that could be pharmacologically manipulated to either reduce the viability of the fungus during infection, or to unleash the fungicidal potential of current antifungal drugs. In this review, we summarize the emerging evidence that the ability of A. fumigatus to sustain viability during stress relies heavily on an adaptive signaling pathway known as the unfolded protein response (UPR), thereby exposing a vulnerability in this fungus that has strong potential for future therapeutic intervention.
Keywords: Aspergillus fumigatus; ER stress; HacA; IreA; UPR; cell death.
© 2022 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: David Askew reports financial support was provided by National Institute of Health.
Figures
References
-
- Abad A., Fernández-Molina J.V., Bikandi J., Ramírez A., Margareto J., Sendino J., Hernando F.L., Pontón J., Garaizar J., Rementeria A. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev. Iberoam. Micol. 2010;27:155–182. 10.1016/j.riam.2010.10.003. - PubMed
-
- Barac A., Kosmidis C., Alastruey-Izquierdo A., Salzer H.J.F., CPAnet Chronic pulmonary aspergillosis update: A year in review. Med. Mycol. 2019;57:S104–S109. 10.1093/mmy/myy070. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

