The involvement of FATP1 regulating skeletal muscle fat deposition in stressed broilers was affected by fatty acid substrates
- PMID: 35909684
- PMCID: PMC9334852
- DOI: 10.3389/fvets.2022.965894
The involvement of FATP1 regulating skeletal muscle fat deposition in stressed broilers was affected by fatty acid substrates
Abstract
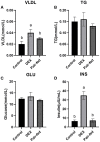
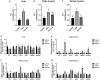
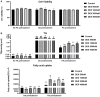
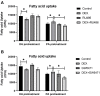
Fatty acid transport protein 1 (FATP1), plays a major role in the transport and uptake of fatty acids into cells. The effect of FATP1 on the regulation of skeletal muscle fat uptake and deposition in stressed broiler chickens was investigated both in vivo and in vitro, and the effect of different fatty acid substrates were also included. Dexamethasone (DEX), a synthetic glucocorticoid (GCs), was employed to induce a hyper glucocorticoid milieu and simulate stress. The in vivo results showed that DEX would increase the mRNA expression of FATP1 and fat deposition in muscle tissues (P < 0.05), the very-low-density lipoprotein (VLDL) and insulin (INS) levels were significantly increased in the plasma by DEX (P < 0.05), and the mRNA levels of the glucocorticoid receptor (GR), adiponectin receptor (ADPNR) and peroxisomal proliferator-activated receptor α (PPARα) in thigh were also up-regulated by DEX (P < 0.05). In vitro experiment, DEX did not affect the myoblast fat deposition and PPARα and FATP1 expressions without the external fatty acid (P > 0.05). Under PA pre-treatment, both myoblast fatty acid uptake and fat deposition were promoted by DEX treatment (P < 0.05), and the effects of DEX on the gene expressions of GR, ADPNR, PPARα and FATP1 were upregulated first and then downregulated as the dose of DEX increases; while under OA pre-treatment, the myoblast fat deposition was not affected by DEX (P > 0.05), the fatty acid uptake was decreased by DEX at 500 nM compared to control (P < 0.05). When GR and PPARα were, respectively inhibited by specific inhibitors RU486 and GW6471, the effects of DEX on fatty acid uptake were reversed for PA pre-treated myoblasts (P < 0.05) but not for OA pre-treated myoblasts (P > 0.05). These results indicate that FATP1 regulation by GCs was affected by fatty acid substrate - saturated fatty acids were favorable for fat uptake and deposition, while unsaturated fatty acids were not. GCs may affect the ADPNR-PPARα-FATP1 pathway by binding to its receptors, thus regulating the uptake of saturated fatty acids into myoblasts.
Keywords: FATP1; broiler chicken; fat deposition; skeletal muscle; stress.
Copyright © 2022 Wang, Jiao, Zhao, Lin and Wang.
Figures

Similar articles
-
Dexamethasone facilitates lipid accumulation and mild feed restriction improves fatty acids oxidation in skeletal muscle of broiler chicks (Gallus gallus domesticus).Comp Biochem Physiol C Toxicol Pharmacol. 2010 May;151(4):447-54. doi: 10.1016/j.cbpc.2010.01.010. Epub 2010 Feb 4. Comp Biochem Physiol C Toxicol Pharmacol. 2010. PMID: 20138241
-
Effects of fatty acid treatments on the dexamethasone-induced intramuscular lipid accumulation in chickens.PLoS One. 2012;7(5):e36663. doi: 10.1371/journal.pone.0036663. Epub 2012 May 18. PLoS One. 2012. PMID: 22623960 Free PMC article.
-
Increasing skeletal muscle fatty acid transport protein 1 (FATP1) targets fatty acids to oxidation and does not predispose mice to diet-induced insulin resistance.Diabetologia. 2011 Jun;54(6):1457-67. doi: 10.1007/s00125-011-2114-8. Epub 2011 Mar 26. Diabetologia. 2011. PMID: 21442160
-
Skeletal muscle fatty acids shift from oxidation to storage upon dexamethasone treatment in chickens.Gen Comp Endocrinol. 2012 Dec 1;179(3):319-30. doi: 10.1016/j.ygcen.2012.09.013. Epub 2012 Oct 2. Gen Comp Endocrinol. 2012. PMID: 23036730
-
Contribution of FAT/CD36 to the regulation of skeletal muscle fatty acid oxidation: an overview.Acta Physiol (Oxf). 2008 Dec;194(4):293-309. doi: 10.1111/j.1748-1716.2008.01878.x. Epub 2008 Jul 9. Acta Physiol (Oxf). 2008. PMID: 18510711 Review.
Cited by
-
Genomic Insights into Molecular Regulation Mechanisms of Intramuscular Fat Deposition in Chicken.Genes (Basel). 2023 Dec 10;14(12):2197. doi: 10.3390/genes14122197. Genes (Basel). 2023. PMID: 38137019 Free PMC article. Review.
-
AI-Based Homology Modelling of Fatty Acid Transport Protein 1 Using AlphaFold: Structural Elucidation and Molecular Dynamics Exploration.Biomolecules. 2023 Nov 20;13(11):1670. doi: 10.3390/biom13111670. Biomolecules. 2023. PMID: 38002353 Free PMC article.
-
Effects of main active components of rosemary on growth performance, meat quality and lipid metabolism in finishing pigs.Anim Nutr. 2023 Sep 20;15:341-349. doi: 10.1016/j.aninu.2023.05.015. eCollection 2023 Dec. Anim Nutr. 2023. PMID: 38053801 Free PMC article.
References
-
- Deeb N, Cahaner A. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 3 growth rate and water consumption of broiler progeny from weight-selected versus non-selected parents under normal and high ambient temperatures. Poult Sci. (2002) 81:293–301. 10.1093/ps/81.3.293 - DOI - PubMed
-
- Houshyar H, Galigniana MD, Pratt WB, Woods JH. Differential responsivity of the hypothalamic-pituitary-adrenal axis to glucocorticoid negative-feedback and corticotropin releasing hormone in rats undergoing morphine withdrawal: possible mechanisms involved in facilitated and attenuated stress responses. J Neuroendocrinol. (2001) 13:875–86. 10.1046/j.1365-2826.2001.00714.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

