Nuciferine Regulates Immune Function and Gut Microbiota in DSS-Induced Ulcerative Colitis
- PMID: 35909691
- PMCID: PMC9328756
- DOI: 10.3389/fvets.2022.939377
Nuciferine Regulates Immune Function and Gut Microbiota in DSS-Induced Ulcerative Colitis
Abstract
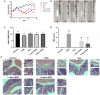
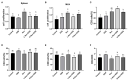
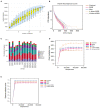
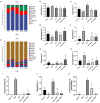
Nuciferine, a major aporphine alkaloid obtained from the leaves of Nelumbo nucifera, exhibits anti-cancer and anti-inflammatory properties; however, its protective effects against inflammatory bowel diseases (IBD) has never been explored. In this study, an ulcerative colitis (UC) model was established in BALb/c mice by the continuous administration of 5% dextran sulfate sodium (DSS) in drinking water for 1 week. From day 8 to day 14, the DSS-treated mice were divided into a high-dose and a low-dose nuciferine treatment group and were intraperitoneally injected with the corresponding dose of the drug. Body weight loss, disease activity index (DAI), and colon length were measured. Histological changes were observed using hematoxylin and eosin staining. T lymphocyte proliferation was assessed by MTT assay. The ratio of CD3+, CD4+, CD8+, Th1, Th2, Th17, and Treg cells were estimated by flow cytometry. Finally, 16S rRNA sequencing was performed to compare the composition and relative abundance of the gut microbiota among the different treatment groups. The results showed that nuciferine treatment led to a significant improvement in symptoms, such as histological injury and colon shortening in mice with DSS-induced UC. Nuciferine treatment improved the Th1/Th2 and Treg/Th17 balance in the DSS-induced IBD model, as well as the composition of the intestinal microflora. At the phylum level, compared with the control group, the abundance of Firmicutes and Actinobacteriota was decreased in the model group, whereas that of Bacteroidetes increased. Meanwhile, at the genus level, compared with the control group, the numbers of the genera Lachnospiraceae_Clostridium, Bilophila and Halomonas reduced in the model group, while those of Bacteroides, Parabacteroides, and Paraprevotella increased. Notably, nuciferine administration reversed this DSS-induced gut dysbiosis. These results indicated that nuciferine modulates gut microbiota homeostasis and immune function in mice with DSS-induced UC.
Keywords: T cells; Th1/Th2 cells; Treg/Th17 cells; gut microbiota; immune function; nuciferine; ulcerative colitis.
Copyright © 2022 Zhu, Zhao, Huang, Li, Yu, Zhang, Liu, Yan, Xia, Guo, Liu, Yang and Zeng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
2,3,5,4'-Tetrahydroxystilbene-2-O-β-D-glucoside, a major bioactive component from Polygoni multiflori Radix (Heshouwu) suppresses DSS induced acute colitis in BALb/c mice by modulating gut microbiota.Biomed Pharmacother. 2021 May;137:111420. doi: 10.1016/j.biopha.2021.111420. Epub 2021 Feb 23. Biomed Pharmacother. 2021. PMID: 33761623
-
Rhubarb Peony Decoction ameliorates ulcerative colitis in mice by regulating gut microbiota to restoring Th17/Treg balance.J Ethnopharmacol. 2019 Mar 1;231:39-49. doi: 10.1016/j.jep.2018.08.033. Epub 2018 Aug 28. J Ethnopharmacol. 2019. PMID: 30170079
-
Anti-tumor necrosis factor α therapy associates to type 17 helper T lymphocytes immunological shift and significant microbial changes in dextran sodium sulphate colitis.World J Gastroenterol. 2019 Mar 28;25(12):1465-1477. doi: 10.3748/wjg.v25.i12.1465. World J Gastroenterol. 2019. PMID: 30948910 Free PMC article.
-
Juglone regulates gut microbiota and Th17/Treg balance in DSS-induced ulcerative colitis.Int Immunopharmacol. 2021 Aug;97:107683. doi: 10.1016/j.intimp.2021.107683. Epub 2021 Apr 26. Int Immunopharmacol. 2021. PMID: 33915494
-
Structure-activity relationship, bioactivities, molecular mechanisms, and clinical application of nuciferine on inflammation-related diseases.Pharmacol Res. 2023 Jul;193:106820. doi: 10.1016/j.phrs.2023.106820. Epub 2023 Jun 12. Pharmacol Res. 2023. PMID: 37315822 Review.
Cited by
-
The role of complex interactions between the intestinal flora and host in regulating intestinal homeostasis and inflammatory bowel disease.Front Microbiol. 2023 Jun 14;14:1188455. doi: 10.3389/fmicb.2023.1188455. eCollection 2023. Front Microbiol. 2023. PMID: 37389342 Free PMC article. Review.
-
Natural aporphine alkaloids: A comprehensive review of phytochemistry, pharmacokinetics, anticancer activities, and clinical application.J Adv Res. 2024 Sep;63:231-253. doi: 10.1016/j.jare.2023.11.003. Epub 2023 Nov 5. J Adv Res. 2024. PMID: 37935346 Free PMC article. Review.
-
Bear Bile Powder Improves Ulcerative Colitis by Protecting the Intestinal Mechanical Barrier and Regulating Intestinal Flora.Curr Pharm Des. 2024;30(19):1530-1540. doi: 10.2174/0113816128294893240403074953. Curr Pharm Des. 2024. PMID: 38676524
-
T helper cell 17/regulatory T cell balance regulates ulcerative colitis and the therapeutic role of natural plant components: a review.Front Med (Lausanne). 2025 Mar 24;11:1502849. doi: 10.3389/fmed.2024.1502849. eCollection 2024. Front Med (Lausanne). 2025. PMID: 40196424 Free PMC article. Review.
-
Water-Soluble Se-Containing Proteins from Chicken Alleviate DSS-Induced Ulcerative Colitis in Mice via Inhibiting TLR4/MyD88 Pathway and Protecting the Goblet Cell Pathway.Biol Trace Elem Res. 2024 Aug;202(8):3767-3780. doi: 10.1007/s12011-023-03952-1. Epub 2023 Nov 11. Biol Trace Elem Res. 2024. PMID: 37950138
References
-
- Grosu IA, Pistol GC, Marin DE, Cismileanu A, Palade LM, Taranu I. Effects of dietary grape seed meal bioactive compounds on the colonic microbiota of weaned piglets with dextran sodium sulfate-induced colitis used as an inflammatory model. Front Vet Sci. (2020) 7:31. 10.3389/fvets.2020.00031 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

