Quarantine and serial testing for variants of SARS-CoV-2 with benefits of vaccination and boosting on consequent control of COVID-19
- PMID: 35909795
- PMCID: PMC9335027
- DOI: 10.1093/pnasnexus/pgac100
Quarantine and serial testing for variants of SARS-CoV-2 with benefits of vaccination and boosting on consequent control of COVID-19
Abstract
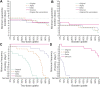
Quarantine and serial testing strategies for a disease depend principally on its incubation period and infectiousness profile. In the context of COVID-19, these primary public health tools must be modulated with successive SARS CoV-2 variants of concern that dominate transmission. Our analysis shows that (1) vaccination status of an individual makes little difference to the determination of the appropriate quarantine duration of an infected case, whereas vaccination coverage of the population can have a substantial effect on this duration, (2) successive variants can challenge disease control efforts by their earlier and increased transmission in the disease time course relative to prior variants, and (3) sufficient vaccine boosting of a population substantially aids the suppression of local transmission through frequent serial testing. For instance, with Omicron, increasing immunity through vaccination and boosters-for instance with 100% of the population is fully immunized and at least 24% having received a third dose-can reduce quarantine durations by up to 2 d, as well as substantially aid in the repression of outbreaks through serial testing. Our analysis highlights the paramount importance of maintaining high population immunity, preferably by booster uptake, and the role of quarantine and testing to control the spread of SARS CoV-2.
Keywords: disease time course; omicron; public health; severe acute respiratory syndrome; variants of concern.
© The Author(s) 2022. Published by Oxford University Press on behalf of National Academy of Sciences.
Figures

References
-
- Wells CR, et al. 2022. Comparative analyses of FDA EUA-approved rapid antigen tests and RT-PCR for COVID-19 quarantine and surveillance-based isolation. medRxiv. https://www.medrxiv.org/content/10.1101/2021.08.23.21262499v4, (accessed 2022 Feb 15). 2021.08.23.21262499 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

