Suppression of AGTR1 Induces Cellular Senescence in Hepatocellular Carcinoma Through Inactivating ERK Signaling
- PMID: 35910032
- PMCID: PMC9326343
- DOI: 10.3389/fbioe.2022.929979
Suppression of AGTR1 Induces Cellular Senescence in Hepatocellular Carcinoma Through Inactivating ERK Signaling
Abstract
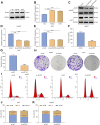
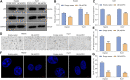
Objective: Cellular senescence is an effective barrier against tumorigenesis. Hence, it is of significance to characterize key features of cellular senescence and the induction of senescence in hepatocellular carcinoma (HCC) cells via pharmacological interventions. Our study determined the biological roles as well as mechanisms of angiotensin II type I receptor (AGTR1) on cellular senescence in HCC. Methods: Lentivirus vector-mediated overexpression or knockdown of AGTR1 was conducted in HCC cells, respectively. A volume of 8 μM sorafenib was used to induce cellular senescence, and ERK was activated by 30 ng/ml ERK agonist EGF. Proliferation was evaluated via clone formation assay. HCC cell senescence was examined by flow cytometry for cell cycle, senescence-associated β-galactosidase (SA-β-gal) staining, and senescence-associated heterochromatin foci (SAHF) analysis. AGTR1, p53, p21, extracellular signal-regulated kinase (ERK), and p-ERK expression were assessed through Western blot or immunofluorescence. Results: AGTR1-knockout HCC cells displayed the attenuated proliferative capacity, G2-M phase arrest, increased expression of p53 and p21, and elevated percentages of SA-β-gal- and SAHF-positive cells. In sorafenib-exposed HCC cells, overexpressed AGTR1 enhanced the proliferative capacity and alleviated G2-M phase arrest as well as decreased p53 and p21 expression and the proportions of SA-β-gal- and SAHF-positive cells. Moreover, AGTR1 knockdown attenuated the activity of p-ERK in HCC cells, and ERK agonist ameliorated AGTR1 knockdown-induced cellular senescence. Conclusion: This study demonstrates that suppression of AGTR1 induces cellular senescence in HCC through inactivating ERK signaling. The significant synergistic effect of AGTR1 suppression and sorafenib might represent a potential combination therapy for HCC.
Keywords: AGTR1; ERK signaling; cellular senescence; hepatocellular carcinoma; sorafenib.
Copyright © 2022 Wang, Cui, Gong, Xu, Huang and Tang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Inhibition of Twist1-mediated invasion by Chk2 promotes premature senescence in p53-defective cancer cells.Cell Death Differ. 2017 Jul;24(7):1275-1287. doi: 10.1038/cdd.2017.70. Epub 2017 May 12. Cell Death Differ. 2017. PMID: 28498365 Free PMC article.
-
Atorvastatin-induced senescence of hepatocellular carcinoma is mediated by downregulation of hTERT through the suppression of the IL-6/STAT3 pathway.Cell Death Discov. 2020 Mar 30;6:17. doi: 10.1038/s41420-020-0252-9. eCollection 2020. Cell Death Discov. 2020. PMID: 32257389 Free PMC article.
-
circLARP4 induces cellular senescence through regulating miR-761/RUNX3/p53/p21 signaling in hepatocellular carcinoma.Cancer Sci. 2019 Feb;110(2):568-581. doi: 10.1111/cas.13901. Epub 2019 Jan 4. Cancer Sci. 2019. PMID: 30520539 Free PMC article.
-
SIRT1 overexpression antagonizes cellular senescence with activated ERK/S6k1 signaling in human diploid fibroblasts.PLoS One. 2008 Mar 5;3(3):e1710. doi: 10.1371/journal.pone.0001710. PLoS One. 2008. PMID: 18320031 Free PMC article.
-
Senescence-Associated β-Galactosidase Detection in Pathology.Diagnostics (Basel). 2022 Sep 25;12(10):2309. doi: 10.3390/diagnostics12102309. Diagnostics (Basel). 2022. PMID: 36291998 Free PMC article. Review.
Cited by
-
Transcriptome analysis reveals the alleviating effect of Polysaccharide of Atractylodes macrocephala Koidz on thymic involution in Magang geese.Poult Sci. 2025 Jun;104(6):105155. doi: 10.1016/j.psj.2025.105155. Epub 2025 Apr 12. Poult Sci. 2025. PMID: 40245540 Free PMC article.
-
The role of angiotensinogen, angiotensin II receptor type I and type II receptor polymorphisms (AGTR2 rs11091046, AGT rs699, AGTR1 rs5186, and AGT rs4762) in multiple myeloma.Discov Oncol. 2025 Aug 21;16(1):1588. doi: 10.1007/s12672-025-03443-w. Discov Oncol. 2025. PMID: 40836159 Free PMC article.
-
A cellular senescence-related genes model allows for prognosis and treatment stratification of hepatocellular carcinoma: A bioinformatics analysis and experimental verification.Front Genet. 2023 Jan 12;13:1099148. doi: 10.3389/fgene.2022.1099148. eCollection 2022. Front Genet. 2023. PMID: 36712870 Free PMC article.
-
Regulation of cellular senescence in tumor progression and therapeutic targeting: mechanisms and pathways.Mol Cancer. 2025 Apr 2;24(1):106. doi: 10.1186/s12943-025-02284-z. Mol Cancer. 2025. PMID: 40170077 Free PMC article. Review.
References
-
- Berger M. D., Stintzing S., Heinemann V., Yang D., Cao S., Sunakawa Y., et al. (2017). Impact of Genetic Variations in the MAPK Signaling Pathway on Outcome in Metastatic Colorectal Cancer Patients Treated with First-Line FOLFIRI and Bevacizumab: Data from FIRE-3 and TRIBE Trials. Ann. Oncol. 28 (11), 2780–2785. 10.1093/annonc/mdx412 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

