Caffeic Acid Phenethyl Ester as a DHODH Inhibitor and Its Synergistic Anticancer Properties in Combination with 5-Fluorouracil in a Breast Cancer Cell Line
- PMID: 35910085
- PMCID: PMC9329448
- DOI: 10.2147/JEP.S365159
Caffeic Acid Phenethyl Ester as a DHODH Inhibitor and Its Synergistic Anticancer Properties in Combination with 5-Fluorouracil in a Breast Cancer Cell Line
Abstract
Introduction: A combination of chemotherapy agents is the best choice in breast cancer treatment to increase the patient survival rate. 5-fluorouracil (5-FU) is one of the drugs applied in combination with other drugs to control and delay development of cancer cells. Nevertheless, the occurrence of multidrug resistance and dose-limiting cytotoxicity have limited the efficacy of 5-FU treatment. Therefore, the discovery of new anti-breast cancer drugs should be pursued.
Objective: To study potency of a promising naturally derived compound, caffeic acid phenethyl ester (CAPE), for breast cancer treatment in single and combination with 5-FU.
Methods: Cytotoxicity of CAPE, 5-FU, and 5-FU+CAPE was studied by in vitro MTT experiment in MCF-7 cell line, and RT-PCR analysis was used to evaluate the change in gene expression due to the treatment. Moreover, an enzymatic assay and molecular docking analysis were applied to evaluate the possible mechanism of substance-induced apoptosis.
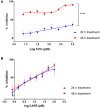
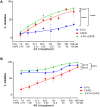
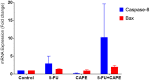
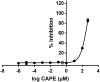
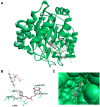
Results: The study revealed that a single treatment of CAPE showed cytotoxicity with IC50 6.6 ± 1.0 µM and 6.5 ± 2.9 µM at 24 h and 48 h, respectively. Meanwhile, 5-FU showed cytostatic activity. The 5-FU + CAPE has a synergistic effect at 24 h treatment with a CI = 0.5 and an additive effect at 48 h treatment with CI = 1.0. CAPE was also found to enhances the mRNA expression of caspase-8 and BAX within 6 hours in combination with 5-FU compared to 5-FU treatment alone. Our study reveals a new mechanism of CAPE which is related to the inhibition of human dihydroorotate dehydrogenase (HsDHODH) with an IC50 of 120.7 ± 6.8 µM, by bound to the ubiquinone-binding site of the enzyme and could be responsible for inducing extrinsic and intrinsic apoptosis.
Conclusion: This study demonstrated the cytotoxicity of CAPE potential to induce apoptosis of breast cancer MCF-7 cell line single and cytotoxic-cytostatic combination with 5-FU. Therefore, further studies to develop CAPE and its derivatives will be required to discover new candidates for breast cancer agents.
Keywords: 5-fluorouracil; DHODH; MCF-7; breast cancer; caffeic acid phenethyl ester; dihydroorotate dehydrogenase.
© 2022 Amalia et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Pulsed Electromagnetic Field Enhances Caffeic Acid Phenethyl Ester-induced Death of MCF-7 Breast Cancer Cells.Anticancer Res. 2024 Jun;44(6):2407-2415. doi: 10.21873/anticanres.17047. Anticancer Res. 2024. PMID: 38821617
-
Comparison of Two Components of Propolis: Caffeic Acid (CA) and Caffeic Acid Phenethyl Ester (CAPE) Induce Apoptosis and Cell Cycle Arrest of Breast Cancer Cells MDA-MB-231.Molecules. 2017 Sep 15;22(9):1554. doi: 10.3390/molecules22091554. Molecules. 2017. PMID: 28926932 Free PMC article.
-
Caffeic Acid Versus Caffeic Acid Phenethyl Ester in the Treatment of Breast Cancer MCF-7 Cells: Migration Rate Inhibition.Integr Cancer Ther. 2018 Dec;17(4):1247-1259. doi: 10.1177/1534735418801521. Epub 2018 Sep 24. Integr Cancer Ther. 2018. PMID: 30246565 Free PMC article.
-
The anticancer mechanism of caffeic acid phenethyl ester (CAPE): review of melanomas, lung and prostate cancers.Eur Rev Med Pharmacol Sci. 2012 Dec;16(15):2064-8. Eur Rev Med Pharmacol Sci. 2012. PMID: 23280020 Review.
-
Combination of Conventional Drugs with Biocompounds Derived from Cinnamic Acid: A Promising Option for Breast Cancer Therapy.Biomedicines. 2023 Jan 19;11(2):275. doi: 10.3390/biomedicines11020275. Biomedicines. 2023. PMID: 36830811 Free PMC article. Review.
Cited by
-
Differential Apoptotic Effects of Bee Product Mixtures on Normal and Cancer Hepatic Cells.Antioxidants (Basel). 2023 Mar 2;12(3):615. doi: 10.3390/antiox12030615. Antioxidants (Basel). 2023. PMID: 36978864 Free PMC article.
-
The DHODH inhibitor teriflunomide impedes cell proliferation and enhances chemosensitivity to daunorubicin (DNR) in T-cell acute lymphoblastic leukemia.Ann Hematol. 2024 Dec;103(12):5449-5460. doi: 10.1007/s00277-024-05998-0. Epub 2024 Oct 8. Ann Hematol. 2024. PMID: 39377943
-
DHODH: a promising target in the treatment of T-cell acute lymphoblastic leukemia.Blood Adv. 2023 Nov 14;7(21):6685-6701. doi: 10.1182/bloodadvances.2023010337. Blood Adv. 2023. PMID: 37648673 Free PMC article.
-
Caffeic Acid Phenethyl Ester: A Potential Therapeutic Cancer Agent?Curr Med Chem. 2024;31(41):6760-6774. doi: 10.2174/0109298673252993230921073502. Curr Med Chem. 2024. PMID: 37933215 Review.
-
Anticancer potential of hydroxycinnamic acids: mechanisms, bioavailability, and therapeutic applications.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jan;398(1):469-495. doi: 10.1007/s00210-024-03396-x. Epub 2024 Aug 30. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39212736 Review.
References
-
- World Health Organization. Breast cancer; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/breast-cancer. Accessed July 13, 2022.
-
- Amalia E. Diantini A, Subarnas A. Overview of current and future targets of breast cancer medicines. J Pharm Sci Res. 2019;11:2385–2397.
-
- Sharder A, Rabbani G, Chowdhury HK. Molecular basis of drug interactions of methotrexate, cyclophosphamide and 5-fluorouracil as chemotherapeutic agents in cancer. Biomed Res Ther. 2015;2:196–206.
-
- World Health Organization. Guidelines for management of breast cancer. EMRO Technical Publication Series 31. World Health Organization; 2006.
LinkOut - more resources
Full Text Sources
Research Materials

