Effect of Sublethal Copper Overload on Cholesterol De Novo Synthesis in Undifferentiated Neuronal Cells
- PMID: 35910134
- PMCID: PMC9330139
- DOI: 10.1021/acsomega.2c00703
Effect of Sublethal Copper Overload on Cholesterol De Novo Synthesis in Undifferentiated Neuronal Cells
Abstract
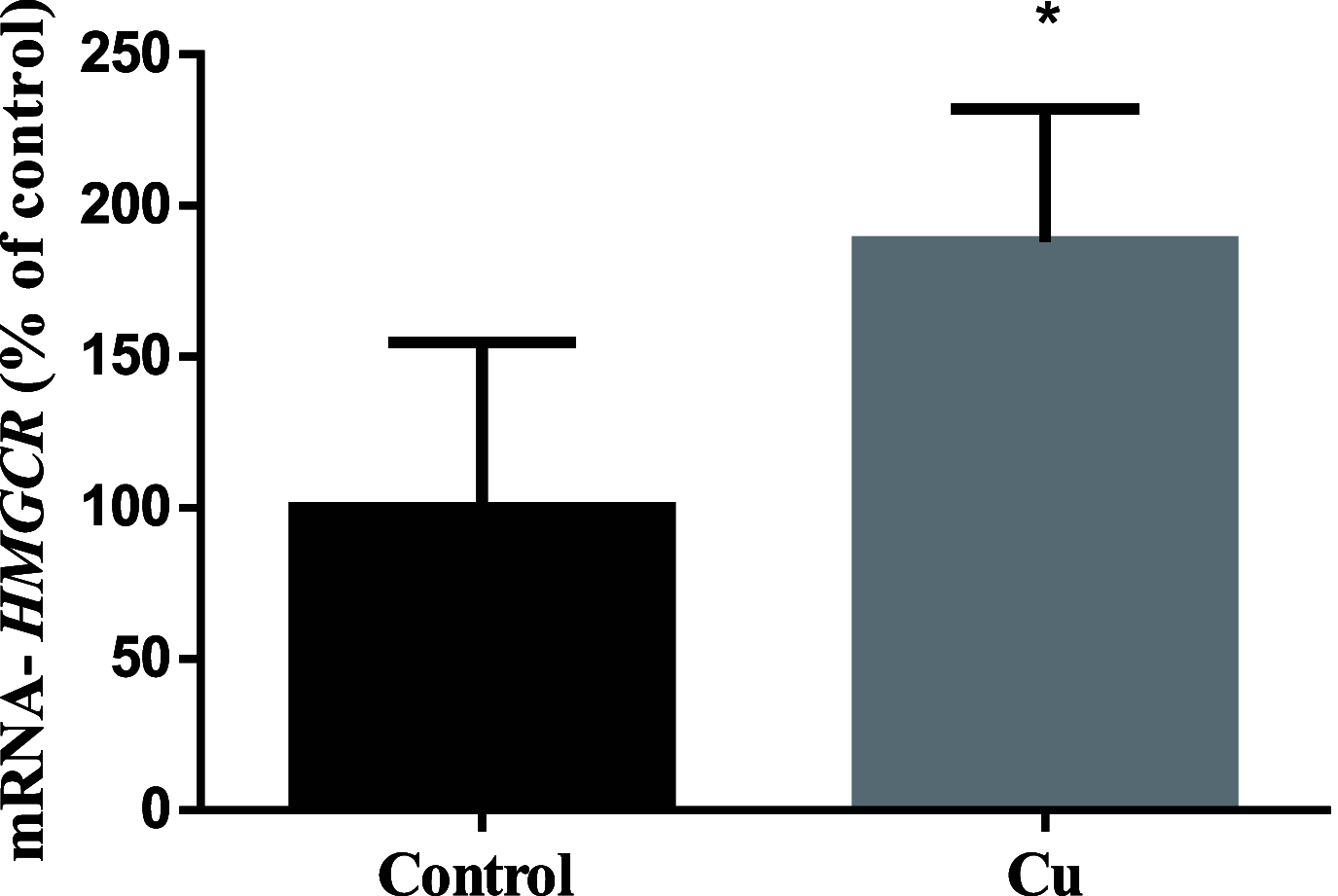
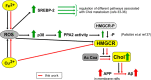
Although copper (Cu) is an essential trace metal for cells, it can induce harmful effects as it participates in the Fenton reaction. Involuntary exposure to Cu overload is much more common than expected and has been linked with neurodegeneration, particularly with Alzheimer's disease (AD) evidenced by a positive correlation between free Cu in plasma and the severity of the disease. It has been suggested that Cu imbalance alters cholesterol (Chol) homeostasis and that high membrane Chol promotes the amyloidogenic processing of the amyloid precursor protein (APP) secreting the β-amyloid (Aβ) peptide. Despite the wide knowledge on the effects of Cu in mature brain metabolism, the consequence of its overload on immature neurons remains unknown. Therefore, we used an undifferentiated human neuroblastoma cell line (SH-SY5Y) to analyze the effect of sublethal concentrations of Cu on 1- de novo Chol synthesis and membrane distribution; 2-APP levels in cells and its distribution in membrane rafts; 3-the levels of Aβ in the culture medium. Our results demonstrated that Cu increases reactive oxygen species (ROS) and favors Chol de novo synthesis in both ROS-dependent and independent manners. Also, at least part of these effects was due to the activation of 3-hydroxy-3-methyl glutaryl CoA reductase (HMGCR). In addition, Cu increases the Chol/PL ratio in the cellular membranes, specifically Chol content in membrane rafts. We found no changes in total APP cell levels; however, its presence in membrane rafts increases with the consequent increase of Aβ in the culture medium. We conclude that Cu overload favors Chol de novo synthesis in both ROS-dependent and independent manners, being at least in part, responsible for the high Chol levels found in the cell membrane and membrane rafts. These may promote the redistribution of APP into the rafts, favoring the amyloidogenic processing of this protein and increasing the levels of Aβ.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Halliwell B.; Gutteridge J. M. C.. Free Radicals in Biology and Medicine; Oxford University Press, 2015.
LinkOut - more resources
Full Text Sources

