Perampanel as First Add-On Therapy in Patients with Focal-Onset Seizures in the FAME Trial: Post hoc Analyses of Efficacy and Safety Related to Maintenance Dose and Background Antiepileptic Drug Therapy
- PMID: 35910326
- PMCID: PMC9289376
- DOI: 10.14581/jer.22003
Perampanel as First Add-On Therapy in Patients with Focal-Onset Seizures in the FAME Trial: Post hoc Analyses of Efficacy and Safety Related to Maintenance Dose and Background Antiepileptic Drug Therapy
Abstract
Background and purpose: FAME (Fycompa® as first Add-on to Monotherapy in patients with Epilepsy; NCT02726074), a previously reported single-arm, phase IV study, showed that perampanel improved seizure control as first add-on to failed anti-seizure medication (ASM) monotherapy in 85 South Korean patients aged ≥12 years with focal-onset seizures (FOS) with/without focal to bilateral tonic-clonic seizures. We present results of three post hoc analyses of FAME that further assessed the efficacy and safety of perampanel.
Methods: Patients were stratified by low- (4, 6 mg/day) versus high- (8, 10, 12 mg/day) dose maintenance perampanel, perampanel added to first- versus second-line ASM monotherapy, and concomitant background ASM monotherapy and perampanel dose. The primary endpoint was the proportion of patients with a ≥50% reduction in total seizure frequency during the 24-week maintenance period. Safety was assessed by the descriptive incidence of treatment-emergent adverse events (TEAEs).
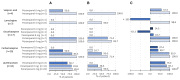
Results: In post hoc analyses, 50% responder rates were significantly higher for low- versus high-dose maintenance perampanel (88.6% vs. 40.0%; p<0.001) and when added to first- versus second-line ASM monotherapy (83.5% vs. 33.3%; p=0.013). By concomitant background ASM and perampanel maintenance dose, 50% responder rates were 100% for perampanel 4 mg/day added to carbamazepine, oxcarbazepine, lamotrigine, or valproic acid, and 85% when added to levetiracetam. Add-on perampanel improved 75% and seizure-free responder rates, and median percent changes from baseline seizure frequency per 28 days. Perampanel was well tolerated when added to ASM monotherapy, with dizziness being the most common TEAE.
Conclusions: Post hoc analyses of FAME provide supportive data for the use of perampanel as an effective and well-tolerated first add-on treatment to a broad spectrum of ASM monotherapies in patients with FOS.
Keywords: AMPA receptor; Perampanel; Seizures; focal; generalized.
Copyright © 2022 Korean Epilepsy Society.
Conflict of interest statement
Conflict of Interest Ji Woong Lee and Min Young Kim are employees of Eisai Korea Inc. All other authors have no conflict of interest to declare.
Figures


Similar articles
-
Efficacy and safety of perampanel as early add-on therapy in Chinese patients with focal-onset seizures: a multicenter, open-label, single-arm study.Front Neurol. 2023 Aug 30;14:1236046. doi: 10.3389/fneur.2023.1236046. eCollection 2023. Front Neurol. 2023. PMID: 37712083 Free PMC article.
-
Perampanel as First Adjunctive Treatment in Patients with Focal-Onset Seizures in the FAME Study: Post hoc Analyses of Dose-Related Efficacy, Safety and Clinical Factors Associated with Response.J Epilepsy Res. 2022 Jun 30;12(1):6-12. doi: 10.14581/jer.22002. eCollection 2022 Jun. J Epilepsy Res. 2022. PMID: 35910330 Free PMC article.
-
First add-on perampanel for focal-onset seizures: An open-label, prospective study.Acta Neurol Scand. 2020 Feb;141(2):132-140. doi: 10.1111/ane.13197. Epub 2019 Dec 4. Acta Neurol Scand. 2020. PMID: 31745975 Free PMC article. Clinical Trial.
-
Perampanel as monotherapy and adjunctive therapy for focal onset seizures, focal to bilateral tonic-clonic seizures and as adjunctive therapy of generalized onset tonic-clonic seizures.Expert Rev Neurother. 2019 Jan;19(1):5-16. doi: 10.1080/14737175.2019.1555474. Epub 2018 Dec 18. Expert Rev Neurother. 2019. PMID: 30560703 Review.
-
Perampanel, an AMPA receptor antagonist: From clinical research to practice in clinical settings.Acta Neurol Scand. 2018 Apr;137(4):378-391. doi: 10.1111/ane.12879. Epub 2017 Dec 7. Acta Neurol Scand. 2018. PMID: 29214650 Review.
Cited by
-
Efficacy and safety of perampanel as early add-on therapy in Chinese patients with focal-onset seizures: a multicenter, open-label, single-arm study.Front Neurol. 2023 Aug 30;14:1236046. doi: 10.3389/fneur.2023.1236046. eCollection 2023. Front Neurol. 2023. PMID: 37712083 Free PMC article.
References
-
- National Institute for Health and Care Excellence (NICE) Epilepsies: diagnosis and management [Internet] London: NICE; 2012. [cited 2021 May 12]. Available at : https://www.nice.org.uk/guidance/CG137 . - PubMed
-
- Byun JI, Kim DW, Kim KT, et al. Treatment of epilepsy in adults: expert opinion in South Korea. Epilepsy Behav. 2020;105:106942. - PubMed
-
- Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58:522–30. - PubMed
LinkOut - more resources
Full Text Sources

