Use of Tox21 Screening Data to Evaluate the COVID-19 Drug Candidates for Their Potential Toxic Effects and Related Pathways
- PMID: 35910344
- PMCID: PMC9333127
- DOI: 10.3389/fphar.2022.935399
Use of Tox21 Screening Data to Evaluate the COVID-19 Drug Candidates for Their Potential Toxic Effects and Related Pathways
Abstract
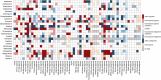
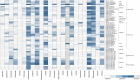
Currently, various potential therapeutic agents for coronavirus disease-2019 (COVID-19), a global pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), are being investigated worldwide mainly through the drug repurposing approach. Several anti-viral, anti-bacterial, anti-malarial, and anti-inflammatory drugs were employed in randomized trials and observational studies for developing new therapeutics for COVID-19. Although an increasing number of repurposed drugs have shown anti-SARS-CoV-2 activities in vitro, so far only remdesivir has been approved by the US FDA to treat COVID-19, and several other drugs approved for Emergency Use Authorization, including sotrovimab, tocilizumab, baricitinib, paxlovid, molnupiravir, and other potential strategies to develop safe and effective therapeutics for SARS-CoV-2 infection are still underway. Many drugs employed as anti-viral may exert unwanted side effects (i.e., toxicity) via unknown mechanisms. To quickly assess these drugs for their potential toxicological effects and mechanisms, we used the Tox21 in vitro assay datasets generated from screening ∼10,000 compounds consisting of approved drugs and environmental chemicals against multiple cellular targets and pathways. Here we summarize the toxicological profiles of small molecule drugs that are currently under clinical trials for the treatment of COVID-19 based on their in vitro activities against various targets and cellular signaling pathways.
Keywords: COVID-19; coronavirus; drugs; high throughput screening; in vitro assay.
Copyright © 2022 Sakamuru, Huang and Xia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Recent advances in small-molecular therapeutics for COVID-19.Precis Clin Med. 2022 Sep 24;5(4):pbac024. doi: 10.1093/pcmedi/pbac024. eCollection 2022 Dec. Precis Clin Med. 2022. PMID: 36268466 Free PMC article. Review.
-
Therapeutic strategies for COVID-19: progress and lessons learned.Nat Rev Drug Discov. 2023 Jun;22(6):449-475. doi: 10.1038/s41573-023-00672-y. Epub 2023 Apr 19. Nat Rev Drug Discov. 2023. PMID: 37076602 Free PMC article. Review.
-
Repurposing Therapeutics for Potential Treatment of SARS-CoV-2: A Review.Viruses. 2020 Jun 30;12(7):705. doi: 10.3390/v12070705. Viruses. 2020. PMID: 32629804 Free PMC article. Review.
-
Drug repurposing in COVID-19: A review with past, present and future.Metabol Open. 2021 Dec;12:100121. doi: 10.1016/j.metop.2021.100121. Epub 2021 Aug 26. Metabol Open. 2021. PMID: 34462734 Free PMC article.
-
Discovery of Potent SARS-CoV-2 Inhibitors from Approved Antiviral Drugs via Docking and Virtual Screening.Comb Chem High Throughput Screen. 2021;24(3):441-454. doi: 10.2174/1386207323999200730205447. Comb Chem High Throughput Screen. 2021. PMID: 32748740
Cited by
-
Development and validation of CYP26A1 inhibition assay for high-throughput screening.Biotechnol J. 2024 Jun;19(6):e2300659. doi: 10.1002/biot.202300659. Biotechnol J. 2024. PMID: 38863121 Free PMC article.
-
Discovery of a Potential Allosteric Site in the SARS-CoV-2 Spike Protein and Targeting Allosteric Inhibitor to Stabilize the RBD Down State using a Computational Approach.Curr Comput Aided Drug Des. 2024;20(6):784-797. doi: 10.2174/1573409919666230726142418. Curr Comput Aided Drug Des. 2024. PMID: 37493168
-
SARS-CoV-2 omicron variants are susceptible in vitro to Artemisia annua hot water extracts.J Ethnopharmacol. 2023 May 23;308:116291. doi: 10.1016/j.jep.2023.116291. Epub 2023 Feb 18. J Ethnopharmacol. 2023. PMID: 36804200 Free PMC article.
-
Bridging imaging-based in vitro methods from biomedical research to regulatory toxicology.Arch Toxicol. 2025 Apr;99(4):1271-1285. doi: 10.1007/s00204-024-03922-z. Epub 2025 Feb 13. Arch Toxicol. 2025. PMID: 39945818 Free PMC article. Review.
-
SARS-CoV-2 omicron variants succumb in vitro to Artemisia annua hot water extracts.bioRxiv [Preprint]. 2022 Jul 25:2022.07.22.501141. doi: 10.1101/2022.07.22.501141. bioRxiv. 2022. Update in: J Ethnopharmacol. 2023 May 23;308:116291. doi: 10.1016/j.jep.2023.116291. PMID: 35923322 Free PMC article. Updated. Preprint.
References
-
- Abd-Elsalam S., Noor R. A., Badawi R., Khalaf M., Esmail E. S., Soliman S., et al. (2021). Clinical Study Evaluating the Efficacy of Ivermectin in COVID-19 Treatment: A Randomized Controlled Study. J. Med. Virol. 93 (10), 5833–5838. 10.1002/jmv.27122 PubMed Abstract | 10.1002/jmv.27122 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Abdulamir A. S., Gorial F. I., Saadi S. J., Maulood M. F., Hashim H. A., Alnuaimi A. S., et al. (2021). A Randomised Controlled Trial of Effectiveness and Safety of Niclosamide as Add on Therapy to the Standard of Care Measures in COVID-19 Management. Ann. Med. Surg. (Lond) 69, 102779. 10.1016/j.amsu.2021.102779 PubMed Abstract | 10.1016/j.amsu.2021.102779 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Ader F., Peiffer-Smadja N., Poissy J., Bouscambert-Duchamp M., Belhadi D., Diallo A., et al. (2021). An Open-Label Randomized Controlled Trial of the Effect of Lopinavir/ritonavir, Lopinavir/ritonavir Plus IFN-β-1a and Hydroxychloroquine in Hospitalized Patients with COVID-19. Clin. Microbiol. Infect. 27 (12), 1826–1837. 10.1016/j.cmi.2021.05.020 PubMed Abstract | 10.1016/j.cmi.2021.05.020 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Al-Bari M. A. (2015). Chloroquine Analogues in Drug Discovery: New Directions of Uses, Mechanisms of Actions and Toxic Manifestations from Malaria to Multifarious Diseases. J. Antimicrob. Chemother. 70 (6), 1608–1621. 10.1093/jac/dkv018 PubMed Abstract | 10.1093/jac/dkv018 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Alexaki V. I., Henneicke H. (2021). The Role of Glucocorticoids in the Management of COVID-19. Horm. Metab. Res. 53 (1), 9–15. 10.1055/a-1300-2550 PubMed Abstract | 10.1055/a-1300-2550 | Google Scholar - DOI - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

