Pharmacokinetic Characterization and Comparative Bioavailability of an Innovative Orodispersible Fixed-Dose Combination of Ivermectin and Albendazole: A Single Dose, Open Label, Sequence Randomized, Crossover Clinical Trial in Healthy Volunteers
- PMID: 35910353
- PMCID: PMC9329971
- DOI: 10.3389/fphar.2022.914886
Pharmacokinetic Characterization and Comparative Bioavailability of an Innovative Orodispersible Fixed-Dose Combination of Ivermectin and Albendazole: A Single Dose, Open Label, Sequence Randomized, Crossover Clinical Trial in Healthy Volunteers
Abstract
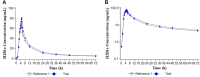
Soil-transmitted helminths are intestinal worm diseases transmitted through the soil. Available treatments are albendazole and/or ivermectin. The co-administration of existing drugs is an appropriate strategy. A fixed-dose combination adds practical advantages mainly considering mass drug administration. The aim is to characterize pharmacokinetics and to evaluate the comparative bioavailability of an innovative fixed-dose combination of ivermectin/albendazole 18/400 mg compared with the marketed references. Seventy-eight healthy volunteers were included in this laboratory-blinded, randomized, three-treatment, three-period crossover study. Each subject received a single dose of ivermectin/albendazole 18/400 mg (1 tablet); ivermectin 3 mg (6 tablets); and albendazole 400 mg (1 tablet). Serial blood samples for the pharmacokinetic analysis were obtained pre-dose and up to 72 h post-dose. Plasma concentrations of ivermectin H2B1a, ivermectin H2B1b, albendazole, and albendazole sulfoxide were analyzed by LC-MS/MS. Pharmacokinetic parameters were estimated by a non-compartmental analysis and bioavailability compared through a bioequivalence analysis. Safety and tolerability were assessed throughout the study. Main pharmacokinetic parameters of the fixed combination were estimated for both, ivermectin [Cmax (mean, confidence interval): 86.40 (30.42-39.23) ng/ml; AUC0-72 (mean, CI): 1,040 (530-1,678) ng·h/mL; tmax (median, min., and max.); 4.50 (2.50-5.50)] and albendazole [Cmax (mean, CI): 22.27 (1.89-111.78) ng/ml; AUC0-72 (mean, CI): 94.65 (11.65-507.78) ng·h/mL; tmax (median, min., and max.): 2.50 (1.00-12.00) h]. The 90% confidence interval of the geometric mean ratios demonstrated the bioequivalence in the case of ivermectin (Cmax: 110.68%-120.49%; AUC0-72: 110.46%-119.60%) but not in the case of albendazole (Cmax: 53.10%-70.34%; AUC0-72: 61.13%-76.54%). The pharmacokinetic profile of a new fixed-dose combination of ivermectin and albendazole was characterized. The bioequivalence versus the reference ivermectin was demonstrated, though bioequivalence versus albendazole was not shown. The three medications analyzed were well tolerated. The results allow the advancement to the next phase of the clinical program to demonstrate efficacy and safety in patients affected by soil-transmitted helminths. Clinical Trial Registration: https://www.clinicaltrialsregister.eu/ctr-search/search/, identifier Nr. 2020-003438-19.
Keywords: albendazole; bioavability; helminitiasis; ivermectin; pharmacokinetics.
Copyright © 2022 Algorta, Krolewiecki, Pinto, Gold and Muñoz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Pharmacokinetics and bioequivalence of Ezetimibe tablet versus Ezetrol®:an open-label, randomized, two-sequence crossover study in healthy Chinese subjects.BMC Pharmacol Toxicol. 2023 Feb 3;24(1):7. doi: 10.1186/s40360-023-00649-y. BMC Pharmacol Toxicol. 2023. PMID: 36737825 Free PMC article. Clinical Trial.
-
Comparative Bioavailability Study of a New Orodispersible Formulation of Ibuprofen Versus Two Existing Oral Tablet Formulations in Healthy Male and Female Volunteers.Clin Ther. 2019 Aug;41(8):1486-1498. doi: 10.1016/j.clinthera.2019.04.040. Epub 2019 Jun 12. Clin Ther. 2019. PMID: 31202508 Clinical Trial.
-
Bioequivalence of fixed dose combination of atorvastatin 10 mg and aspirin 150 mg capsules: a randomized, open-label, single-dose, two-way crossover study in healthy human subjects.Drug Res (Stuttg). 2013 May;63(5):250-7. doi: 10.1055/s-0033-1337931. Epub 2013 Mar 22. Drug Res (Stuttg). 2013. PMID: 23526241 Clinical Trial.
-
Pharmacokinetics and bioequivalence evaluation of gliclazide/metformin combination tablet and equivalent doses of gliclazide and metformin in healthy Korean subjects.Int J Clin Pharmacol Ther. 2009 Dec;47(12):770-9. doi: 10.5414/cpp47770. Int J Clin Pharmacol Ther. 2009. PMID: 19954716 Clinical Trial.
-
Factors associated with variation in single-dose albendazole pharmacokinetics: A systematic review and modelling analysis.PLoS Negl Trop Dis. 2022 Oct 28;16(10):e0010497. doi: 10.1371/journal.pntd.0010497. eCollection 2022 Oct. PLoS Negl Trop Dis. 2022. PMID: 36306320 Free PMC article.
Cited by
-
Variations in Plasma Levels of Orally Administered Ivermectin Could Hamper Its Potential Drug Repositioning: Results of a Bioequivalence Study in Mexican Population.Pharmaceuticals (Basel). 2025 Aug 13;18(8):1193. doi: 10.3390/ph18081193. Pharmaceuticals (Basel). 2025. PMID: 40872584 Free PMC article.
References
-
- Anderson R. M., Turner H. C., Truscott J. E., Hollingsworth T. D., Brooker S. J. (2015). Should the Goal for the Treatment of Soil Transmitted Helminth (STH) Infections Be Changed from Morbidity Control in Children to Community-wide Transmission Elimination? PLoS Negl. Trop. Dis. 9 (8), e0003897. 10.1371/journal.pntd.0003897 - DOI - PMC - PubMed
-
- Awadzi K., Hero M., Opoku N. O., Büttner D. W., Coventry P. A., Prime M. A., et al. (1994). The Chemotherapy of Onchocerciasis XVII. A Clinical Evaluation of Albendazole in Patients with Onchocerciasis; Effects of Food and Pretreatment with Ivermectin on Drug Response and Pharmacokinetics. Trop. Med. Parasitol. 45, 203–208. - PubMed
LinkOut - more resources
Full Text Sources

