Determination of the Relative Potency of Norepinephrine and Phenylephrine Given as Infusions for Preventing Hypotension During Combined Spinal-Epidural Anesthesia for Cesarean Delivery: A Randomized Up-And-Down Sequential Allocation Study
- PMID: 35910385
- PMCID: PMC9330490
- DOI: 10.3389/fphar.2022.942005
Determination of the Relative Potency of Norepinephrine and Phenylephrine Given as Infusions for Preventing Hypotension During Combined Spinal-Epidural Anesthesia for Cesarean Delivery: A Randomized Up-And-Down Sequential Allocation Study
Abstract
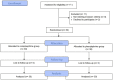
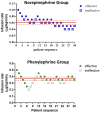
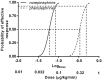
Purpose: The relative potency of norepinephrine and phenylephrine given as boluses to treat hypotension during spinal anesthesia for cesarean delivery has been reported but few data are available for infusions. This study aimed to determine the relative potency of norepinephrine and phenylephrine when given by infusion for preventing hypotension during combined spinal-epidural anesthesia for cesarean delivery. Methods: This was a prospective, randomized, double-blind, up-and-down sequential allocation study. Patients were randomly allocated to receive a prophylactic infusion of norepinephrine or phenylephrine started immediately after induction of anesthesia. The first patients received either norepinephrine 0.1 μg/kg/min or phenylephrine 0.5 μg/kg/min. An effective infusion rate was defined when no hypotension occurred before delivery. For each subsequent patient, the norepinephrine infusion rate was decreased or increased by 0.01 μg/kg/min or the phenylephrine infusion rate was decreased or increased by 0.05 μg/kg/min according to whether the infusion was effective or ineffective respectively in the previous patient. Values for the infusion rate that was effective in preventing hypotension in 50% of patients (ED50) for norepinephrine and phenylephrine were estimated using up-and-down sequential analysis and relative potency was estimated. Probit regression was used as a backup and sensitivity analysis. Results: The ED50 values for norepinephrine and phenylephrine calculated by the up-and-down method were 0.061 (95% CI 0.054-0.068) μg/kg/min and 0.368 (95% CI 0.343-0.393) μg/kg/min respectively. The estimated relative potency ratio for ED50 for norepinephrine to phenylephrine was 6.03:1 (95% CI 5.26:1 to 6.98:1). Conclusion: Under the conditions of this study, norepinephrine given by infusion was about 6 times more potent than phenylephrine. This information is useful for clinical practice and further comparative studies of norepinephrine versus phenylephrine. Clinical Trial Registration: http://www.chictr.org.cn/showproj.aspx, identifier [ChiCTR2200056237].
Keywords: anaesthesia; cesarean section; infusions; intravenous; norepinephrine; phenylephrine; spinal.
Copyright © 2022 Qian, Zhao, Deng, Wang, Xiao, Shen and Yao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Effect of crystalloid solution co-loading infusion rate on the dose requirements of prophylactic phenylephrine for preventing hypotension following combined spinal-epidural anesthesia for cesarean delivery.BMC Pregnancy Childbirth. 2024 Nov 12;24(1):743. doi: 10.1186/s12884-024-06937-7. BMC Pregnancy Childbirth. 2024. PMID: 39533243 Free PMC article. Clinical Trial.
-
A Prospective, Randomized, Double-Blinded Study of the Effect of Intravenous Ondansetron on the Effective Dose in 50% of Subjects of Prophylactic Phenylephrine Infusions for Preventing Spinal Anesthesia-Induced Hypotension During Cesarean Delivery.Anesth Analg. 2020 Aug;131(2):564-569. doi: 10.1213/ANE.0000000000004534. Anesth Analg. 2020. PMID: 31725021 Clinical Trial.
-
Prophylactic Norepinephrine and Phenylephrine Boluses to Prevent Postspinal Anesthesia Hypotension During Cesarean Section: A Randomized Sequential Allocation Dose-Finding Study.Drug Des Devel Ther. 2023 May 23;17:1547-1555. doi: 10.2147/DDDT.S406671. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37249928 Free PMC article. Clinical Trial.
-
Prophylactic intravenous norepinephrine for the prevention of hypotension during spinal anesthesia for elective cesarean section: a systematic review and dose-response meta-analysis of randomized controlled trials.Front Pharmacol. 2023 Sep 19;14:1247214. doi: 10.3389/fphar.2023.1247214. eCollection 2023. Front Pharmacol. 2023. PMID: 37795034 Free PMC article.
-
The Efficacy and Safety of Norepinephrine and Its Feasibility as a Replacement for Phenylephrine to Manage Maternal Hypotension during Elective Cesarean Delivery under Spinal Anesthesia.Biomed Res Int. 2018 Dec 31;2018:1869189. doi: 10.1155/2018/1869189. eCollection 2018. Biomed Res Int. 2018. PMID: 30687737 Free PMC article. Review.
Cited by
-
Up-and-Down Determination of Different Crystalloid Coload Volumes on the ED 90 of Prophylactic Norepinephrine Infusion for Preventing Postspinal Anesthesia Hypotension During Cesarean Section.Drug Des Devel Ther. 2024 Jun 26;18:2609-2616. doi: 10.2147/DDDT.S460436. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38947222 Free PMC article. Clinical Trial.
-
Neuraxial anaesthesia-induced hypotension during Caesarean section.BJA Educ. 2024 Apr;24(4):113-120. doi: 10.1016/j.bjae.2024.01.003. Epub 2024 Feb 15. BJA Educ. 2024. PMID: 38481416 Free PMC article. Review. No abstract available.
-
Effects of Prophylactic Infusion of Equivalent Doses of Norepinephrine and Phenylephrine in Preventing Spinal Anesthesia-Induced Hypotension During Cesarean Delivery on Fetal and Maternal Outcomes: A Dual-Center, Non-Inferiority Controlled Trial.Drug Des Devel Ther. 2025 Jun 17;19:5143-5152. doi: 10.2147/DDDT.S514091. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40557054 Free PMC article. Clinical Trial.
-
Determination of ED90s of Phenylephrine and Norepinephrine Infusion for Prevention of Spinal Anesthesia-Induced Hypotension in Patients with Preeclampsia During Cesarean Delivery.Drug Des Devel Ther. 2024 Jul 5;18:2813-2821. doi: 10.2147/DDDT.S467072. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38984209 Free PMC article. Clinical Trial.
-
Prophylactic infusion of norepinephrine does not affect the rostral spread of spinal anesthesia in pregnancy: a prospective, randomized, double-blinded study.Front Pharmacol. 2024 Jan 9;14:1340452. doi: 10.3389/fphar.2023.1340452. eCollection 2023. Front Pharmacol. 2024. PMID: 38264521 Free PMC article.
References
-
- Fieller E. C. (1940). The Biological Standardization of Insulin. Suppl. J. R. Stat. Soc. 7, 1–64. 10.2307/2983630 - DOI
LinkOut - more resources
Full Text Sources

