"In Litero" Screening: Retrospective Evaluation of Clinical Evidence to Establish a Reference List of Human Chemical Respiratory Sensitizers
- PMID: 35910543
- PMCID: PMC9335368
- DOI: 10.3389/ftox.2022.916370
"In Litero" Screening: Retrospective Evaluation of Clinical Evidence to Establish a Reference List of Human Chemical Respiratory Sensitizers
Abstract
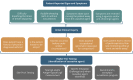
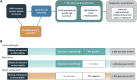
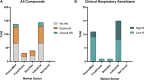
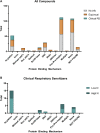
Despite decades of investigation, test methods to identify respiratory sensitizers remain an unmet regulatory need. In order to support the evaluation of New Approach Methodologies in development, we sought to establish a reference set of low molecular weight respiratory sensitizers based on case reports of occupational asthma. In this context, we have developed an "in litero" approach to identify cases of low molecular weight chemical exposures leading to respiratory sensitization in clinical literature. We utilized the EPA-developed Abstract Sifter literature review tool to maximize the retrieval of publications relevant to respiratory effects in humans for each chemical in a list of chemicals suspected of inducing respiratory sensitization. The literature retrieved for each of these candidate chemicals was sifted to identify relevant case reports and studies, and then evaluated by applying defined selection criteria. Clinical diagnostic criteria were defined around exposure history, respiratory effects, and specific immune response to conclusively demonstrate occupational asthma as a result of sensitization, rather than irritation. This approach successfully identified 28 chemicals that can be considered as human respiratory sensitizers and used to evaluate the performance of NAMs as part of a weight of evidence approach to identify novel respiratory sensitizers. Further, these results have immediate implications for the development and refinement of predictive tools to distinguish between skin and respiratory sensitizers. A comparison of the protein binding mechanisms of our identified "in litero" clinical respiratory sensitizers shows that acylation is a prevalent protein binding mechanism, in contrast to Michael addition and Schiff base formation common to skin sensitizers. Overall, this approach provides an exemplary method to evaluate and apply human data as part of the weight of evidence when establishing reference chemical lists.
Keywords: adverse outcome pathway; allergic asthma; chemical allergens; clinical reference list; new approach methodologies; occupational asthma; respiratory sensitisation; respiratory sensitization.
Copyright © 2022 Ponder, Rajagopal, Singal, Baker, Patlewicz, Roggen, Cochrane and Sullivan.
Conflict of interest statement
Authors RR and SC were employed by Unilever. Author MS was employed by AeroTox Consulting Services, LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Responses of an In Vitro Coculture Alveolar Model for the Prediction of Respiratory Sensitizers (ALIsens®) Following Exposure to Skin Sensitizers and Non-Sensitizers.Toxics. 2024 Dec 31;13(1):29. doi: 10.3390/toxics13010029. Toxics. 2024. PMID: 39853027 Free PMC article.
-
Identifying a reference list of respiratory sensitizers for the evaluation of novel approaches to study respiratory sensitization.Crit Rev Toxicol. 2021 Nov;51(10):792-804. doi: 10.1080/10408444.2021.2024142. Epub 2022 Feb 10. Crit Rev Toxicol. 2021. PMID: 35142253 Review.
-
Use and limitations of clinical data in the identification and classification of low molecular weight chemicals (LMWCs) as respiratory sensitizers: recommendations for improvement.Crit Rev Toxicol. 2025 Jan;55(1):27-54. doi: 10.1080/10408444.2024.2433222. Epub 2025 Jan 9. Crit Rev Toxicol. 2025. PMID: 39785825 Review.
-
How to assess respiratory sensitization of low molecular weight chemicals?Int J Hyg Environ Health. 2020 Apr;225:113469. doi: 10.1016/j.ijheh.2020.113469. Epub 2020 Feb 12. Int J Hyg Environ Health. 2020. PMID: 32058937 Review.
-
Prediction of chemical respiratory sensitizers using GARD, a novel in vitro assay based on a genomic biomarker signature.PLoS One. 2015 Mar 11;10(3):e0118808. doi: 10.1371/journal.pone.0118808. eCollection 2015. PLoS One. 2015. PMID: 25760038 Free PMC article.
Cited by
-
Safety Implications of Modulating Nuclear Receptors: A Comprehensive Analysis from Non-Clinical and Clinical Perspectives.Pharmaceuticals (Basel). 2024 Jul 3;17(7):875. doi: 10.3390/ph17070875. Pharmaceuticals (Basel). 2024. PMID: 39065726 Free PMC article.
-
Chemical respiratory sensitization-Current status of mechanistic understanding, knowledge gaps and possible identification methods of sensitizers.Front Toxicol. 2024 Jul 29;6:1331803. doi: 10.3389/ftox.2024.1331803. eCollection 2024. Front Toxicol. 2024. PMID: 39135743 Free PMC article. Review.
-
Refining the Amino Reactivity-Based Identification of Respiratory Sensitizers.Chem Res Toxicol. 2025 Jun 16;38(6):1046-1060. doi: 10.1021/acs.chemrestox.4c00545. Epub 2025 May 29. Chem Res Toxicol. 2025. PMID: 40439452 Free PMC article.
-
Formaldehyde and asthma: a plausibility?Arch Toxicol. 2025 Mar;99(3):865-885. doi: 10.1007/s00204-024-03946-5. Epub 2025 Jan 19. Arch Toxicol. 2025. PMID: 39828805 Review.
-
Responses of an In Vitro Coculture Alveolar Model for the Prediction of Respiratory Sensitizers (ALIsens®) Following Exposure to Skin Sensitizers and Non-Sensitizers.Toxics. 2024 Dec 31;13(1):29. doi: 10.3390/toxics13010029. Toxics. 2024. PMID: 39853027 Free PMC article.
References
-
- Baba K., Yagi T., Niwa S., Sakakibara A., Hattori T., Koishikawa I., et al. (2000). Measurement of Formaldehyde-specific IgE Antibodies in Adult Asthmatics. Arerugi 49 (5), 404–411. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

