Evidence that resistin acts on the mechanical responses of the mouse gastric fundus
- PMID: 35910552
- PMCID: PMC9334560
- DOI: 10.3389/fphys.2022.930197
Evidence that resistin acts on the mechanical responses of the mouse gastric fundus
Abstract
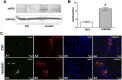
Resistin, among its several actions, has been reported to exert central anorexigenic effects in rodents. Some adipokines which centrally modulate food intake have also been reported to affect the activity of gastric smooth muscle, whose motor responses represent a source of peripheral signals implicated in the control of the hunger-satiety cycle through the gut-brain axis. On this basis, in the present experiments, we investigated whether resistin too could affect the mechanical responses in the mouse longitudinal gastric fundal strips. Electrical field stimulation (EFS) elicited tetrodotoxin- and atropine-sensitive contractile responses. Resistin reduced the amplitude of the EFS-induced contractile responses. This effect was no longer detected in the presence of L-NNA, a nitric oxide (NO) synthesis inhibitor. Resistin did not influence the direct muscular response to methacholine. In the presence of carbachol and guanethidine, EFS elicited inhibitory responses whose amplitude was increased by resistin. L-NNA abolished the inhibitory responses evoked by EFS, indicating their nitrergic nature. In the presence of L-NNA, resistin did not have any effect on the EFS-evoked inhibitory responses. Western blot and immunofluorescence analysis revealed a significant increase in neuronal nitric oxide synthase (nNOS) expression in neurons of the myenteric plexus following resistin exposure. In conclusion, the present results offer the first evidence that resistin acts on the gastric fundus, likely through a modulatory action on the nitrergic neurotransmission.
Keywords: gastric motility; neuromodulation; nitric oxide; resistin; satiety signals.
Copyright © 2022 Idrizaj, Garella, Nistri, Squecco and Baccari.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Aitken-Buck H. M., Babakr A. A., Fomison-Nurse I. C., van Hout I., Davis P. J., Bunton R. W., et al. (2020). Inotropic and lusitropic, but not arrhythmogenic, effects of adipocytokine resistin on human atrial myocardium. Am. J. Physiol. Endocrinol. Metab. 319, 540–547. 10.1152/ajpendo.00202.2020 - DOI - PubMed
LinkOut - more resources
Full Text Sources

