Plasma Levels of Acyl-Carnitines and Carboxylic Acids Correlate With Cardiovascular and Kidney Function in Subjects With Sickle Cell Trait
- PMID: 35910560
- PMCID: PMC9326174
- DOI: 10.3389/fphys.2022.916197
Plasma Levels of Acyl-Carnitines and Carboxylic Acids Correlate With Cardiovascular and Kidney Function in Subjects With Sickle Cell Trait
Abstract
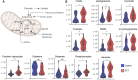
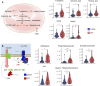
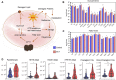
Subjects with sickle cell trait (SCT) carry one copy of mutated β-globin gene at position E6V at the origin of the production of sickle hemoglobin (HbS). Indeed, individuals with SCT have both normal hemoglobin and HbS, in contrast to patients with sickle cell disease who inherited of two copies of the mutated gene. Although SCT is generally benign/asymptomatic, carriers may develop certain adverse outcomes such as renal complications, venous thromboembolism, exercise-induced rhabdomyolysis … However, little is known about whether similar metabolic pathways are affected in individuals with SCT and whether these metabolic derangements, if present, correlate to clinically relevant parameters. In this study, we performed metabolomics analysis of plasma from individuals with sickle cell trait (n = 34) compared to healthy controls (n = 30). Results indicated a significant increase in basal circulating levels of hemolysis markers, mono- (pyruvate, lactate), di- and tri-carboxylates (including all Krebs cycle intermediates), suggestive of systems-wide mitochondrial dysfunction in individuals with SCT. Elevated levels of kynurenines and indoles were observed in SCT samples, along with increases in the levels of oxidative stress markers (advanced glycation and protein-oxidation end-products, malondialdehyde, oxylipins, eicosanoids). Increases in circulating levels of acyl-carnitines and fatty acids were observed, consistent with increased membrane lipid damage in individuals with sickle cell trait. Finally, correlation analyses to clinical co-variates showed that alterations in the aforementioned pathways strongly correlated with clinical measurements of blood viscosity, renal (glomerular filtration rate, microalbuminuria, uremia) and cardiovascular function (carotid-femoral pulse wave velocity, blood pressure).
Keywords: hemoglobin S; lands cycle; metabolome; mitochondria; sickle cell trait.
Copyright © 2022 Nemkov, Skinner, Diaw, Diop, Samb, Connes and D’Alessandro.
Conflict of interest statement
AD and TN are founders of Omix Tehcnologies Inc and Altis Biosciences. AD is also a scientific advisory board member for Hemanext Inc and Forma Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources

