Cutaneous TRPV4 Channels Activate Warmth-Defense Responses in Young and Adult Birds
- PMID: 35910562
- PMCID: PMC9337882
- DOI: 10.3389/fphys.2022.892828
Cutaneous TRPV4 Channels Activate Warmth-Defense Responses in Young and Adult Birds
Abstract
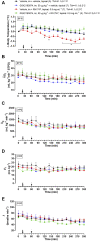
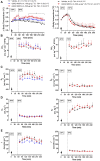
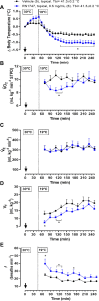
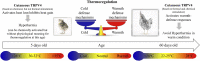
Transient receptor potential vanilloid 4 (TRPV4) channels are sensitive to warm ambient temperatures (Tas), triggering heat loss responses in adult rats in a Tas range of ∼26-30°C. In birds, however, the thermoregulatory role of TRPV4 has never been shown. Here, we hypothesized that stimulation of TRPV4 induces thermolytic responses for body temperature (Tb) maintenance in birds, and that this function is already present in early life, when the Ta range for TRPV4 activation does not represent a warm condition for these animals. We first demonstrated the presence of TRPV4 in the dorsal and ventral skin of chickens (Gallus gallus domesticus) by immunohistochemistry. Then, we evaluated the effects of the TRPV4 agonist, RN1747, and the TRPV4 antagonists, HC067047 and GSK2193874, on Tb and thermoeffectors at different Tas in 5-day-old chicks and 60-day-old adult chickens. For the chicks, RN1747 transiently reduced Tb both in thermoneutrality (31°C) and in a cold Ta for this phase (26°C), which relied on huddling behavior inhibition. The TRPV4 antagonists alone did not affect Tb or thermoeffectors but blocked the Tb decrease and huddling inhibition promoted by RN1747. For the adults, TRPV4 antagonism increased Tb when animals were exposed to 28°C (suprathermoneutral condition for adults), but not to 19°C. In contrast, RN1747 decreased Tb by reducing metabolic rate and activating thermal tachypnea at 19°C, a Ta below the activation range of TRPV4. Our results indicate that peripheral TRPV4 receptors are functional in early life, but may be inhibited at that time when the range of activation (∼26-30°C) represents cold Ta for chicks, and become physiologically relevant for Tb maintenance when the activation Ta range for TRPV4 becomes suprathermoneutral for adult chickens.
Keywords: Metabolism; body temperature; chicken; huddling; peripheral thermoreceptor; thermolysis; thermoregulation.
Copyright © 2022 Cristina-Silva, Amaral-Silva, Santos, Correa, da Silva, Fernandes, da Silva, Gargaglioni, Almeida and Bicego.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Almeida M. C., Hew-Butler T., Soriano R. N., Rao S., Wang W., Wang J., et al. (2012). Pharmacological Blockade of the Cold Receptor TRPM8 Attenuates Autonomic and Behavioral Cold Defenses and Decreases Deep Body Temperature. J. Neurosci. 32, 2086–2099. 10.1523/JNEUROSCI.5606-11.2012 - DOI - PMC - PubMed
-
- Amaral-Silva L., Tazawa H., Bicego K. C., Burggren W. W. (2020). Metabolic and Hematological Responses to Endotoxin-Induced Inflammation in Chicks Experiencing Embryonic TCDD Exposure. Environ. Toxicol. Chem. 39, 2208–2220. - PubMed
LinkOut - more resources
Full Text Sources

