Synergistic Antimicrobial Effect of Antimicrobial Peptides CATH-1, CATH-3, and PMAP-36 With Erythromycin Against Bacterial Pathogens
- PMID: 35910608
- PMCID: PMC9335283
- DOI: 10.3389/fmicb.2022.953720
Synergistic Antimicrobial Effect of Antimicrobial Peptides CATH-1, CATH-3, and PMAP-36 With Erythromycin Against Bacterial Pathogens
Abstract
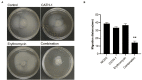
With the increasing bacterial resistance to traditional antibiotics, there is an urgent need for the development of alternative drugs or adjuvants of antibiotics to enhance antibacterial efficiency. The combination of antimicrobial peptides (AMPs) and traditional antibiotics is a potential alternative to enhance antibacterial efficiency. In this study, we investigated the synergistic bactericidal effect of AMPs, including chicken (CATH-1,-2,-3, and -B1), mice (CRAMP), and porcine (PMAP-36 and PR-39) in combination with conventional antibiotics containing ampicillin, tetracycline, gentamicin, and erythromycin against Staphylococcus aureus, Salmonella enteritidis, and Escherichia coli. The results showed that the minimum bactericidal concentration (MBC) of CATH-1,-3 and PMAP-36 was lower than 10 μM, indicating that these three AMPs had good bacterial activity against S. aureus, S. enteritidis, and E. coli. Then, the synergistic antibacterial activity of AMPs and antibiotics combination was determined by the fractional bactericidal concentration index (FBCI). The results showed that the FBCI of AMPs (CATH-1,-3 and PMAP-36) and erythromycin was lower than 0.5 against bacterial pathogens, demonstrating that they had a synergistic bactericidal effect. Furthermore, the time-killing kinetics of AMPs (CATH-1,-3 and PMAP-36) in combination with erythromycin showed that they had a continuous killing effect on bacteria within 3 h. Notably, the combination showed lower hemolytic activity and cytotoxicity to mammal cells compared to erythromycin and peptide alone treatment. In addition, the antibacterial mechanism of CATH-1 and erythromycin combination against E. coli was studied. The results of the scanning electron microscope showed that CATH-1 enhanced the antibacterial activity of erythromycin by increasing the permeability of bacterial cell membrane. Moreover, the results of bacterial migration movement showed that the combination of CATH-1 and erythromycin significantly inhibits the migration of E. coli. Finally, drug resistance analysis was performed and the results showed that CATH-1 delayed the emergence of E. coli resistance to erythromycin. In conclusion, the combination of CATH-1 and erythromycin has synergistic antibacterial activity and reduces the emergence of bacterial drug resistance. Our study provides valuable information to develop AMPs as potential substitutes or adjuvants for traditional antibiotics.
Keywords: antibacterial mechanism; antibiotic resistance; antimicrobial peptides; synergistic antibacterial activity; traditional antibiotics.
Copyright © 2022 Lu, Tian, Chen, Liu, Jia, Hu, Chen, Ye, Peng and Fang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Blodkamp S., Kadlec K., Gutsmann T., Naim H. Y., von Köckritz-Blickwede M., Schwarz S., et al. . (2016). In vitro activity of human and animal cathelicidins against livestock-associated methicillin-resistant Staphylococcus aureus. Vet. Microbiol. 194, 107–111. 10.1016/j.vetmic.2015.09.018 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

