Salmonella Phages Affect the Intestinal Barrier in Chicks by Altering the Composition of Early Intestinal Flora: Association With Time of Phage Use
- PMID: 35910610
- PMCID: PMC9329052
- DOI: 10.3389/fmicb.2022.947640
Salmonella Phages Affect the Intestinal Barrier in Chicks by Altering the Composition of Early Intestinal Flora: Association With Time of Phage Use
Abstract
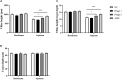
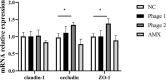
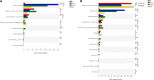
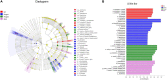
Phages show promise in replacing antibiotics to treat or prevent bacterial diseases in the chicken breeding industry. Chicks are easily affected by their environment during early growth. Thus, this study investigated whether oral phages could affect the intestinal barrier function of chicks with a focus on the cecal microbiome. In a two-week trial, forty one-day-old hens were randomly divided into four groups: (1) NC, negative control; (2) Phage 1, 109 PFU phage/day (days 3-5); (3) Phage 2, 109 PFU phage/day (days 8-10); and (4) AMX, 1 mg/mL amoxicillin/day (days 8-10). High-throughput sequencing results of cecal contents showed that oral administration of phages significantly affected microbial community structure and community composition, and increased the relative abundance of Enterococcus. The number of different species in the Phage 1 group was much higher than that in the Phage 2 group, and differences in alpha and beta diversity also indicated that the magnitude of changes in the composition of the cecal microbiota correlated with the time of phage use. Particularly in the first stage of cecal microbiota development, oral administration of bacteriophages targeting Salmonella may cause substantial changes in chicks, as evidenced by the results of the PICRUSt2 software function prediction, reminding us to be cautious about the time of phage use in chicks and to avoid high oral doses of phages during the first stage. Additionally, the Phage 2 samples not only showed a significant increase in the relative abundance of Bifidobacterium and Subdoligranulum, but also improved the intestinal morphology (jejunum) and increased the mRNA expression level of occludin and ZO-1. We concluded that phages do not directly interact with eukaryotic cells. The enhancement of intestinal barrier function by phages in chicks may be related to changes in the intestinal flora induced by phages. This implies that phages may affect intestinal health by regulating the intestinal flora. This study provides new ideas for phage prevention of intestinal bacterial infections and promotes large-scale application of phages in the poultry industry.
Keywords: Enterococcus; cecal microbiota; chicks; intestinal barrier; phage.
Copyright © 2022 Zhao, Li, Lv, Huang, Tai, Jin, Wang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Systemic Effects of a Phage Cocktail on Healthy Weaned Piglets.Biology (Basel). 2024 Apr 18;13(4):271. doi: 10.3390/biology13040271. Biology (Basel). 2024. PMID: 38666883 Free PMC article.
-
Research Note: Therapeutic effect of a Salmonella phage combination on chicks infected with Salmonella Typhimurium.Poult Sci. 2023 Jul;102(7):102715. doi: 10.1016/j.psj.2023.102715. Epub 2023 Apr 12. Poult Sci. 2023. PMID: 37209652 Free PMC article.
-
Salmonella phage CKT1 significantly relieves the body weight loss of chicks by normalizing the abnormal intestinal microbiome caused by hypervirulent Salmonella Pullorum.Poult Sci. 2022 Mar;101(3):101668. doi: 10.1016/j.psj.2021.101668. Epub 2021 Dec 23. Poult Sci. 2022. PMID: 35063807 Free PMC article.
-
The human oral phageome.Periodontol 2000. 2021 Jun;86(1):79-96. doi: 10.1111/prd.12363. Epub 2021 Mar 10. Periodontol 2000. 2021. PMID: 33690937 Review.
-
Encapsulation and delivery of phage as a novel method for gut flora manipulation in situ: A review.J Control Release. 2023 Jan;353:634-649. doi: 10.1016/j.jconrel.2022.11.048. Epub 2022 Dec 14. J Control Release. 2023. PMID: 36464065 Review.
Cited by
-
Effect of Enrofloxacin on the Microbiome, Metabolome, and Abundance of Antibiotic Resistance Genes in the Chicken Cecum.Microbiol Spectr. 2023 Feb 22;11(2):e0479522. doi: 10.1128/spectrum.04795-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36840593 Free PMC article.
-
Isolation and identification of specific Enterococcus faecalis phage C-3 and G21-7 against Avian pathogenic Escherichia coli and its application to one-day-old geese.Front Microbiol. 2024 Jun 19;15:1385860. doi: 10.3389/fmicb.2024.1385860. eCollection 2024. Front Microbiol. 2024. PMID: 38962142 Free PMC article.
-
Systemic Effects of a Phage Cocktail on Healthy Weaned Piglets.Biology (Basel). 2024 Apr 18;13(4):271. doi: 10.3390/biology13040271. Biology (Basel). 2024. PMID: 38666883 Free PMC article.
-
Antioxidant activity of Jinxuan tea polysaccharide and its protective effect on intestinal injury induced by transport stress in chicks.Front Vet Sci. 2025 Jun 25;12:1610218. doi: 10.3389/fvets.2025.1610218. eCollection 2025. Front Vet Sci. 2025. PMID: 40636806 Free PMC article.
-
Prospects and Challenges of Bacteriophage Substitution for Antibiotics in Livestock and Poultry Production.Biology (Basel). 2024 Jan 4;13(1):28. doi: 10.3390/biology13010028. Biology (Basel). 2024. PMID: 38248459 Free PMC article. Review.
References
-
- Banerjee S., Sar A., Misra A., Pal S., Chakraborty A., Dam B. (2018). Increased productivity in poultry birds by sub-lethal dose of antibiotics is arbitrated by selective enrichment of gut microbiota, particularly short-chain fatty acid producers. Microbiology 164 142–153. 10.1099/mic.0.000597 - DOI - PubMed
LinkOut - more resources
Full Text Sources

