Metabolites From Trypanosome-Infected Cattle as Sensitive Biomarkers for Animal Trypanosomosis
- PMID: 35910617
- PMCID: PMC9329068
- DOI: 10.3389/fmicb.2022.922760
Metabolites From Trypanosome-Infected Cattle as Sensitive Biomarkers for Animal Trypanosomosis
Abstract
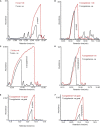
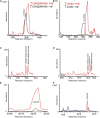
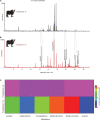
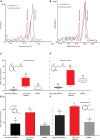
Trypanosomes are important global livestock and human pathogens of public health importance. Elucidating the chemical mechanisms of trypanosome-relevant host interactions can enhance the design and development of a novel, next-generation trypanosomosis diagnostics. However, it is unknown how trypanosome infection affects livestock volatile odors. Here, we show that Trypanosoma congolense and Trypanosoma vivax infections induced dihydro-β- ionone and junenol, while abundance of dihydro-α-ionone, phenolics, p-cresol, and 3-propylphenol significantly elevated in cow urine. These biomarkers of trypanosome infection are conserved in cow breath and the urine metabolites of naturally infected cows, regardless of population, diet, or environment differences. Furthermore, treating trypanosome-infected cows reduced the levels of these indicators back to the pre-infection levels. Finally, we demonstrated that the potential of some specific biomarkers of phenolic origin may be used to detect active trypanosome infections, including low-level infections that are not detectable by microscopy. The sensitivity and specificity of biomarkers detection are suited for rapid, robust, and non-invasive trypanosomosis diagnosis under field conditions.
Keywords: animal trypanosomosis; biomarkers; diagnostic; metabolites; volatile.
Copyright © 2022 Getahun, Ngiela, Makwatta, Ahuya, Simon, Kamau, Torto and Masiga.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Livestock trypanosomosis in Uganda: parasite heterogeneity and anaemia status of naturally infected cattle, goats and pigs.Parasitol Res. 2013 Apr;112(4):1443-50. doi: 10.1007/s00436-013-3275-9. Epub 2013 Jan 24. Parasitol Res. 2013. PMID: 23344247
-
Detection of multiple drug-resistant Trypanosoma congolense populations in village cattle of south-east Mali.Parasit Vectors. 2012 Aug 1;5:155. doi: 10.1186/1756-3305-5-155. Parasit Vectors. 2012. PMID: 22852796 Free PMC article.
-
Trypanosomosis in The Gambia: prevalence in working horses and donkeys detected by whole genome amplification and PCR, and evidence for interactions between trypanosome species.BMC Vet Res. 2008 Feb 20;4:7. doi: 10.1186/1746-6148-4-7. BMC Vet Res. 2008. PMID: 18289378 Free PMC article.
-
Trypanosomosis prevalence in natural field-based infection: insights into systematic review and meta-analysis of studies from 1980 to 2018 on The Gambian ruminants with special emphasis on cattle.Trop Anim Health Prod. 2020 Nov 21;53(1):25. doi: 10.1007/s11250-020-02461-w. Trop Anim Health Prod. 2020. PMID: 33219890
-
Trypanosomosis in goats: current status.Ann N Y Acad Sci. 2006 Oct;1081:300-10. doi: 10.1196/annals.1373.040. Ann N Y Acad Sci. 2006. PMID: 17135529 Review.
Cited by
-
Multimodal interactions in Stomoxys navigation reveal synergy between olfaction and vision.Sci Rep. 2024 Jul 31;14(1):17724. doi: 10.1038/s41598-024-68726-8. Sci Rep. 2024. PMID: 39085483 Free PMC article.
-
Multi-omics analyses reveal rumen microbes and secondary metabolites that are unique to livestock species.mSystems. 2024 Feb 20;9(2):e0122823. doi: 10.1128/msystems.01228-23. Epub 2024 Jan 31. mSystems. 2024. PMID: 38294243 Free PMC article.
-
An Overview of Tsetse Fly Repellents: Identification and Applications.J Chem Ecol. 2024 Nov;50(11):581-592. doi: 10.1007/s10886-024-01527-5. Epub 2024 Jul 8. J Chem Ecol. 2024. PMID: 38976099 Free PMC article. Review.
-
Consistent detection of Trypanosoma brucei but not T. congolense DNA in faeces of experimentally infected cattle.Sci Rep. 2024 Feb 20;14(1):4158. doi: 10.1038/s41598-024-54857-5. Sci Rep. 2024. PMID: 38378867 Free PMC article.
-
Cas10 based 7SL-sRNA diagnostic for the detection of active trypanosomosis.PLoS Negl Trop Dis. 2025 Mar 17;19(3):e0012937. doi: 10.1371/journal.pntd.0012937. eCollection 2025 Mar. PLoS Negl Trop Dis. 2025. PMID: 40096045 Free PMC article.
References
-
- Adams E. R., Malele I. I., Msangi A. R., Gibson W. C. (2006). Trypanosome identification in wild tsetse populations in Tanzania using generic primers to amplify the ribosomal RNA ITS-1 region. Acta Trop. 100 103–109. - PubMed
-
- Alsan M. (2015). The effect of the tsetse fly on African development. Am. Econ. Rev. 105 382–410. 10.1257/aer.20130604 - DOI
LinkOut - more resources
Full Text Sources

