Microbial communities associated with mounds of the Orange-footed scrubfowl Megapodius reinwardt
- PMID: 35910771
- PMCID: PMC9332330
- DOI: 10.7717/peerj.13600
Microbial communities associated with mounds of the Orange-footed scrubfowl Megapodius reinwardt
Abstract
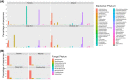
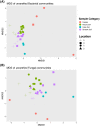
Megapodius reinwardt, the orange-footed scrubfowl, belongs to a small family of birds that inhabits the Indo-Australian region. Megapodes are unique in incubating their eggs in mounds using heat from microbial decomposition of organic materials and solar radiation. Little is known about the microorganisms involved in the decomposition of organic matter in mounds. To determine the source of microbes in the mounds, we used 16S and 18S rRNA gene sequencing to characterize the microbial communities of mound soil, adjacent soil and scrubfowl faeces. We found that the microbial communities of scrubfowl faeces were substantially different from those of the mounds and surrounding soils, suggesting that scrubfowls probably do not use their faeces to inoculate their mounds although a few microbial sequence variants were present in both faeces and mound samples. Further, the mound microbial community structure was significantly different to the adjacent soils. For example, mounds had a high relative abundance of sequence variants belonging to Thermomonosporaceae, a thermophilic soil bacteria family able to degrade cellulose from plant residues. It is not clear whether members of Thermomonosporaceae disproportionately contribute to the generation of heat in the mound, or whether they simply thrive in the warm mound environment created by the metabolic activity of the mound microbial community. The lack of clarity in the literature between designations of heat-producing (thermogenic) and heat-thriving (thermophilic) microbes poses a challenge to understanding the role of specific bacteria and fungi in incubation.
Keywords: Megapode; Microbial communities; Mound; Orange-footed Scrubfowl; Soil microbes; Thermogenesis; Thermomonosporaceae.
© 2022 Cardenas Gomez et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Apprill A, McNally S, Parsons R, Weber L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquatic Microbial Ecology. 2015;75(2):129–137. doi: 10.3354/ame01753. - DOI
-
- Banfield E. Megapode mounds and pits. Emu. 1912;12(4):281–283. doi: 10.1071/MU912278f. - DOI
-
- Beffa T, Blanc M, Marilley L, Fischer JL, Lyon P-F, Aragno M. The Science of Composting. New York: Springer; 1996. Taxonomic and metabolic microbial diversity during composting; pp. 149–161.
-
- Bodor A, Bounedjoum N, Vincze GE, Kis Á.E, Laczi K, Bende G, Szilágyi Á, Kovács T, Perei K, Rákhely G. Challenges of unculturable bacteria: environmental perspectives. Reviews in Environmental Science and Bio/Technology. 2020;19(1):1–22. doi: 10.1007/s11157-020-09522-4. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

