Impairment of autophagy after spinal cord injury potentiates neuroinflammation and motor function deficit in mice
- PMID: 35910787
- PMCID: PMC9330534
- DOI: 10.7150/thno.72713
Impairment of autophagy after spinal cord injury potentiates neuroinflammation and motor function deficit in mice
Abstract
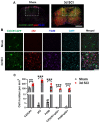
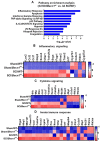
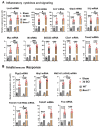
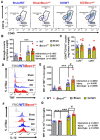
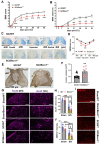
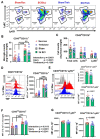
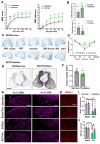
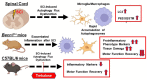
Autophagy is a catabolic process that degrades cytoplasmic constituents and organelles in the lysosome, thus serving an important role in cellular homeostasis and protection against insults. We previously reported that defects in autophagy contribute to neuronal cell damage in traumatic spinal cord injury (SCI). Recent data from other inflammatory models implicate autophagy in regulation of immune and inflammatory responses, with low levels of autophagic flux associated with pro-inflammatory phenotypes. In the present study, we examined the effects of genetically or pharmacologically manipulating autophagy on posttraumatic neuroinflammation and motor function after SCI in mice. Methods: Young adult male C57BL/6, CX3CR1-GFP, autophagy hypomorph Becn1+/- mice, and their wildtype (WT) littermates were subjected to moderate thoracic spinal cord contusion. Neuroinflammation and autophagic flux in the injured spinal cord were assessed using flow cytometry, immunohistochemistry, and NanoString gene expression analysis. Motor function was evaluated with the Basso Mouse Scale and horizontal ladder test. Lesion volume and spared white matter were evaluated by unbiased stereology. To stimulate autophagy, disaccharide trehalose, or sucrose control, was administered in the drinking water immediately after injury and for up to 6 weeks after SCI. Results: Flow cytometry demonstrated dysregulation of autophagic function in both microglia and infiltrating myeloid cells from the injured spinal cord at 3 days post-injury. Transgenic CX3CR1-GFP mice revealed increased autophagosome formation and inhibition of autophagic flux specifically in activated microglia/macrophages. NanoString analysis using the neuroinflammation panel demonstrated increased expression of proinflammatory genes and decreased expression of genes related to neuroprotection in Becn1+/- mice as compared to WT controls at 3 days post-SCI. These findings were further validated by qPCR, wherein we observed significantly higher expression of proinflammatory cytokines. Western blot analysis confirmed higher protein expression of the microglia/macrophage marker IBA-1, inflammasome marker, NLRP3, and innate immune response markers cGAS and STING in Becn1+/- mice at 3 day after SCI. Flow cytometry demonstrated that autophagy deficit did not affect either microglial or myeloid counts at 3 days post-injury, instead resulting in increased microglial production of proinflammatory cytokines. Finally, locomotor function showed significantly worse impairments in Becn1+/- mice up to 6 weeks after SCI, which was accompanied by worsening tissue damage. Conversely, treatment with a naturally occurring autophagy inducer trehalose, reduced protein levels of p62, an adaptor protein targeting cargo to autophagosomes as well as the NLRP3, STING, and IBA-1 at 3 days post-injury. Six weeks of trehalose treatment after SCI led to improved motor function recovery as compared to control group, which was accompanied by reduced tissue damage. Conclusions: Our data indicate that inhibition of autophagy after SCI potentiates pro-inflammatory activation in microglia and is associated with worse functional outcomes. Conversely, increasing autophagy with trehalose, decreased inflammation and improved outcomes. These findings highlight the importance of autophagy in spinal cord microglia and its role in secondary injury after SCI.
Keywords: Autophagy; Beclin-1; Microglia; Neuroinflammation; Spinal cord injury.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
Inhibition of autophagy in microglia and macrophages exacerbates innate immune responses and worsens brain injury outcomes.Autophagy. 2023 Jul;19(7):2026-2044. doi: 10.1080/15548627.2023.2167689. Epub 2023 Jan 18. Autophagy. 2023. PMID: 36652438 Free PMC article.
-
Role of MS4A7 in Regulating Microglial Polarization and Neuroinflammation in Spinal Cord Injury via the cGAS-STING-NLRP3 Axis.CNS Neurosci Ther. 2025 Jun;31(6):e70390. doi: 10.1111/cns.70390. CNS Neurosci Ther. 2025. PMID: 40522023 Free PMC article.
-
Ablation of the integrin CD11b mac-1 limits deleterious responses to traumatic spinal cord injury and improves functional recovery in mice.Res Sq [Preprint]. 2024 Apr 4:rs.3.rs-4196316. doi: 10.21203/rs.3.rs-4196316/v1. Res Sq. 2024. Update in: Cells. 2024 Sep 20;13(18):1584. doi: 10.3390/cells13181584. PMID: 38645238 Free PMC article. Updated. Preprint.
-
The role of autophagy in the regulation of neuroinflammation in acute ischemic stroke (review of literature).Klin Lab Diagn. 2022 Jul 18;67(7):391-398. doi: 10.51620/0869-2084-2022-67-7-391-398. Klin Lab Diagn. 2022. PMID: 35924769 Review. English.
-
Mechanism of effect and therapeutic potential of NLRP3 inflammasome in spinal cord injury.Exp Neurol. 2025 Feb;384:115059. doi: 10.1016/j.expneurol.2024.115059. Epub 2024 Nov 19. Exp Neurol. 2025. PMID: 39571746 Review.
Cited by
-
IPSC-NSCs-derived exosomal let-7b-5p improves motor function after spinal cord Injury by modulating microglial/macrophage pyroptosis.J Nanobiotechnology. 2024 Jul 9;22(1):403. doi: 10.1186/s12951-024-02697-w. J Nanobiotechnology. 2024. PMID: 38982427 Free PMC article.
-
3,4-Dimethoxychalcone, a caloric restriction mimetic, enhances TFEB-mediated autophagy and alleviates pyroptosis and necroptosis after spinal cord injury.Theranostics. 2023 Jan 1;13(2):810-832. doi: 10.7150/thno.78370. eCollection 2023. Theranostics. 2023. PMID: 36632211 Free PMC article.
-
Role of α-synuclein in microglia: autophagy and phagocytosis balance neuroinflammation in Parkinson's disease.Inflamm Res. 2023 Mar;72(3):443-462. doi: 10.1007/s00011-022-01676-x. Epub 2023 Jan 4. Inflamm Res. 2023. PMID: 36598534 Review.
-
The Proteostasis Network: A Global Therapeutic Target for Neuroprotection after Spinal Cord Injury.Cells. 2022 Oct 22;11(21):3339. doi: 10.3390/cells11213339. Cells. 2022. PMID: 36359735 Free PMC article. Review.
-
Phillygenin inhibits neuroinflammation and promotes functional recovery after spinal cord injury via TLR4 inhibition of the NF-κB signaling pathway.J Orthop Translat. 2024 Aug 8;48:133-145. doi: 10.1016/j.jot.2024.07.013. eCollection 2024 Sep. J Orthop Translat. 2024. PMID: 39220679 Free PMC article.
References
-
- Kanno H, Ozawa H, Sekiguchi A, Itoi E. The role of autophagy in spinal cord injury. Autophagy. 2009;5:390–2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

