Turmeric-derived nanovesicles as novel nanobiologics for targeted therapy of ulcerative colitis
- PMID: 35910802
- PMCID: PMC9330521
- DOI: 10.7150/thno.73650
Turmeric-derived nanovesicles as novel nanobiologics for targeted therapy of ulcerative colitis
Abstract
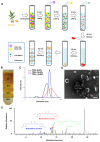
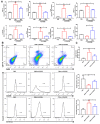
Rationale: Ulcerative colitis (UC), a typical kind of inflammatory bowel disease (IBD), is an idiopathic chronic intestinal inflammation. Conventional therapeutic strategies mainly focus on the rebalance of pro-inflammation and anti-inflammation cytokines, whereas targeting damaged intestinal barriers, imbalanced intestinal microbiota and dysregulated mucosal immune responses in UC remain a big challenge. The objective of this study was to develop turmeric-derived nanovesicles (TNVs) for alleviation of colitis and explore the underlying mechanisms. Methods: TNVs were isolated and purified through differential centrifugation. The targeted ability was evaluated on the dextran sulfate sodium (DSS)-induced mouse model by IVIS imaging system. The anti-inflammation efficacy was studied in lipopolysaccharide (LPS)-induced macrophages and DSS-induced acute and chronic colitic mouse model. In addition, the influence of TNVs on the intestinal microbiota was investigated via 16S rRNA microbiome sequence and the condition of macrophage polarization after TNVs treatment was analyzed by flow cytometry. Results: TNVs were isolated and characterized as nano-size spheroids. The IVIS imaging experiment indicated that orally administrated TNVs could accumulate in the inflamed colon sites and exhibited superior anti-inflammatory activity both in vitro and in vivo. The 16S rRNA sequencing suggested the important role of TNVs in the regulation of gut microbiota. Further, TNVs could promote the transformation of M1 phenotype to M2 macrophages and restore the damaged intestinal epithelium barrier to exert the anti-colitis efficacy. Conclusion: Collectively, oral administration of TNVs exhibited excellent anti-inflammatory efficacy through restoring the damaged intestinal barrier, regulating the gut microbiota and reshaping the macrophage phenotype. This study sheds light on the application of natural exosome-like nanovesicles for the treatment of UC.
Keywords: Fresh herbs; Gut microbiota; Macrophage polarization; Turmeric-derived nanovesicles; Ulcerative colitis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures







References
-
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. - PubMed
-
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152:313–321.e2. - PubMed
-
- Hanauer SB. Update on the etiology, pathogenesis and diagnosis of ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2004;1:26–31. - PubMed
-
- Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 2017;14:269–278. - PubMed
-
- Dubinsky MC. Azathioprine, 6-mercaptopurine in inflammatory bowel disease: pharmacology, efficacy, and safety. Clin Exp Gastroenterol Hepatol. 2004;2:731–743. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

