Subpial delivery of adeno-associated virus 9-synapsin-caveolin-1 (AAV9-SynCav1) preserves motor neuron and neuromuscular junction morphology, motor function, delays disease onset, and extends survival in hSOD1G93A mice
- PMID: 35910808
- PMCID: PMC9330519
- DOI: 10.7150/thno.72614
Subpial delivery of adeno-associated virus 9-synapsin-caveolin-1 (AAV9-SynCav1) preserves motor neuron and neuromuscular junction morphology, motor function, delays disease onset, and extends survival in hSOD1G93A mice
Abstract
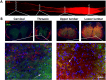
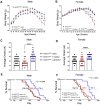
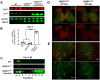
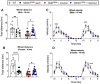
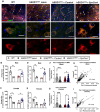
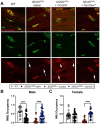
Elevating neuroprotective proteins using adeno-associated virus (AAV)-mediated gene delivery shows great promise in combating devastating neurodegenerative diseases. Amyotrophic lateral sclerosis (ALS) is one such disease resulting from loss of upper and lower motor neurons (MNs) with 90-95% of cases sporadic (SALS) in nature. Due to the unknown etiology of SALS, interventions that afford neuronal protection and preservation are urgently needed. Caveolin-1 (Cav-1), a membrane/lipid rafts (MLRs) scaffolding and neuroprotective protein, and MLR-associated signaling components are decreased in degenerating neurons in postmortem human brains. We previously showed that, when crossing our SynCav1 transgenic mouse (TG) with the mutant human superoxide dismutase 1 (hSOD1G93A) mouse model of ALS, the double transgenic mouse (SynCav1 TG/hSOD1G93A) exhibited better motor function and longer survival. The objective of the current study was to test whether neuron-targeted Cav-1 upregulation in the spinal cord using AAV9-SynCav1 could improve motor function and extend longevity in mutant humanized mouse and rat (hSOD1G93A) models of familial (F)ALS. Methods: Motor function was assessed by voluntary running wheel (RW) in mice and forelimb grip strength (GS) and motor evoked potentials (MEP) in rats. Immunofluorescence (IF) microscopy for choline acetyltransferase (ChAT) was used to assess MN morphology. Neuromuscular junctions (NMJs) were measured by bungarotoxin-a (Btx-a) and synaptophysin IF. Body weight (BW) was measured weekly, and the survival curve was determined by Kaplan-Meier analysis. Results: Following subpial gene delivery to the lumbar spinal cord, male and female hSOD1G93A mice treated with SynCav1 exhibited delayed disease onset, greater running-wheel performance, preserved spinal alpha-motor neuron morphology and NMJ integrity, and 10% increased longevity, independent of affecting expression of the mutant hSOD1G93A protein. Cervical subpial SynCav1 delivery to hSOD1G93A rats preserved forelimb GS and MEPs in the brachial and gastrocnemius muscles. Conclusion: In summary, subpial delivery of SynCav1 protects and preserves spinal motor neurons, and extends longevity in a familial mouse model of ALS without reducing the toxic monogenic component. Furthermore, subpial SynCav1 delivery preserved neuromuscular function in a rat model of FALS. The latter findings strongly indicate the therapeutic applicability of SynCav1 to treat ALS attributed to monogenic (FALS) and potentially in sporadic cases (i.e., SALS).
Keywords: amyotrophic lateral sclerosis; caveolin-1; gene therapy; hSOD1G93A; membrane/lipid raft (MLRs); motor neuron; neuromuscular junction.
© The author(s).
Conflict of interest statement
Competing Interests: D.M.R., H.H.P., P.M.P. and B.P.H. hold equity and are non-paid consultants with Eikonoklastes Therapeutics LLC.
Figures
Similar articles
-
Neuron-targeted caveolin-1 improves neuromuscular function and extends survival in SOD1G93A mice.FASEB J. 2019 Jun;33(6):7545-7554. doi: 10.1096/fj.201802652RR. Epub 2019 Mar 20. FASEB J. 2019. PMID: 30894019 Free PMC article.
-
AAV-NRIP gene therapy ameliorates motor neuron degeneration and muscle atrophy in ALS model mice.Skelet Muscle. 2024 Jul 24;14(1):17. doi: 10.1186/s13395-024-00349-z. Skelet Muscle. 2024. PMID: 39044305 Free PMC article.
-
Nuclear Localization of Human SOD1 in Motor Neurons in Mouse Model and Patient Amyotrophic Lateral Sclerosis: Possible Links to Cholinergic Phenotype, NADPH Oxidase, Oxidative Stress, and DNA Damage.Int J Mol Sci. 2024 Aug 22;25(16):9106. doi: 10.3390/ijms25169106. Int J Mol Sci. 2024. PMID: 39201793 Free PMC article.
-
Transgenic mice with human mutant genes causing Parkinson's disease and amyotrophic lateral sclerosis provide common insight into mechanisms of motor neuron selective vulnerability to degeneration.Rev Neurosci. 2007;18(2):115-36. doi: 10.1515/revneuro.2007.18.2.115. Rev Neurosci. 2007. PMID: 17593875 Review.
-
Inhibitory synaptic regulation of motoneurons: a new target of disease mechanisms in amyotrophic lateral sclerosis.Mol Neurobiol. 2012 Feb;45(1):30-42. doi: 10.1007/s12035-011-8217-x. Epub 2011 Nov 10. Mol Neurobiol. 2012. PMID: 22072396 Free PMC article. Review.
Cited by
-
Physiological and pathological roles of caveolins in the central nervous system.Trends Neurosci. 2024 Aug;47(8):651-664. doi: 10.1016/j.tins.2024.06.003. Epub 2024 Jul 6. Trends Neurosci. 2024. PMID: 38972795 Free PMC article. Review.
-
Role of EPAC1 in chronic pain.Biochem Biophys Rep. 2024 Jan 22;37:101645. doi: 10.1016/j.bbrep.2024.101645. eCollection 2024 Mar. Biochem Biophys Rep. 2024. PMID: 38304575 Free PMC article. Review.
-
Gene therapy breakthroughs in ALS: a beacon of hope for 20% of ALS patients.Transl Neurodegener. 2025 Apr 16;14(1):19. doi: 10.1186/s40035-025-00477-6. Transl Neurodegener. 2025. PMID: 40234983 Free PMC article. Review.
-
Analysis of Feeding Behavior Characteristics in the Cu/Zn Superoxide Dismutase 1 (SOD1) SOD1G93A Mice Model for Amyotrophic Lateral Sclerosis (ALS).Nutrients. 2023 Mar 28;15(7):1651. doi: 10.3390/nu15071651. Nutrients. 2023. PMID: 37049492 Free PMC article.
-
ZC3H15 suppression ameliorates bone cancer pain through inhibiting neuronal oxidative stress and microglial inflammation.Neoplasia. 2025 Mar;61:101123. doi: 10.1016/j.neo.2025.101123. Epub 2025 Feb 4. Neoplasia. 2025. PMID: 39908779 Free PMC article.
References
-
- O'Connor DM, Boulis NM. Gene therapy for neurodegenerative diseases. Trends Mol Med. 2015;21:504–12. - PubMed
-
- Brown RH, Al-Chalabi A. Amyotrophic Lateral Sclerosis. N Engl J Med. 2017;377:162–72. - PubMed
-
- Hardiman O, van den Berg LH. Edaravone: a new treatment for ALS on the horizon? Lancet Neurol. 2017;16:490–1. - PubMed
-
- Ajroud-Driss S, Siddique T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS) Biochim Biophys Acta. 2015;1852:679–84. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

