Biliverdin Reductase A Protects Lens Epithelial Cells against Oxidative Damage and Cellular Senescence in Age-Related Cataract
- PMID: 35910837
- PMCID: PMC9325611
- DOI: 10.1155/2022/5628946
Biliverdin Reductase A Protects Lens Epithelial Cells against Oxidative Damage and Cellular Senescence in Age-Related Cataract
Abstract
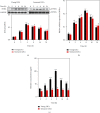
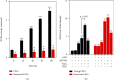
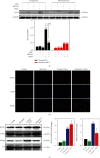
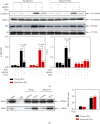
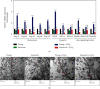
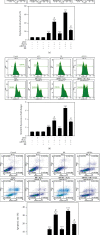
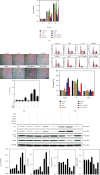
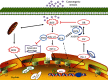
Age-related cataract (ARC) is the common cause of blindness globally. Reactive oxygen species (ROS), one of the greatest contributors to aging process, leads to oxidative damage and senescence of lens epithelial cells (LECs), which are involved in the pathogenesis of ARC. Biliverdin reductase A (BVRA) has ROS-scavenging ability by converting biliverdin (BV) into bilirubin (BR). However, little is known about the protective effect of BVRA against ARC. In the present study, we measured the expression level of BVRA and BR generation in human samples. Then, the antioxidative property of BVRA was compared between the young and senescent LECs upon stress condition. In addition, we evaluated the effect of BVRA on attenuating H2O2-induced premature senescence in LECs. The results showed that the mRNA expression level of BVRA and BR concentration were decreased in both LECs and lens cortex of age-related nuclear cataract. Using the RNA interference technique, we found that BVRA defends LECs against oxidative stress via (i) restoring mitochondrial dysfunction in a BR-dependent manner, (ii) inducing heme oxygenase-1 (HO-1) expression directly, and (iii) promoting phosphorylation of ERK1/2 and nuclear delivery of nuclear factor erythroid 2-related factor 2 (Nrf2). Intriguingly, the antioxidative effect of BVRA was diminished along with the reduced BR concentration and repressed nuclear translocation of BVRA and Nrf2 in senescent LECs, which would be resulted from the decreased BVRA activity and impaired nucleocytoplasmic trafficking. Eventually, we confirmed that BVRA accelerates the G1 phase transition and prevents against H2O2-induced premature senescence in LECs. In summary, BVRA protects LECs against oxidative stress and cellular senescence in ARC by converting BV into BR, inducing HO-1 expression, and activating the ERK/Nrf2 pathway. This trial is registered with ChiCTR2000036059.
Copyright © 2022 Yang Huang et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
HO-1-Mediated Autophagic Restoration Protects Lens Epithelial Cells Against Oxidative Stress and Cellular Senescence.Invest Ophthalmol Vis Sci. 2023 Dec 1;64(15):6. doi: 10.1167/iovs.64.15.6. Invest Ophthalmol Vis Sci. 2023. PMID: 38051262 Free PMC article.
-
Biliverdin/Bilirubin Redox Pair Protects Lens Epithelial Cells against Oxidative Stress in Age-Related Cataract by Regulating NF-κB/iNOS and Nrf2/HO-1 Pathways.Oxid Med Cell Longev. 2022 Apr 15;2022:7299182. doi: 10.1155/2022/7299182. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35480872 Free PMC article.
-
Paired Box Gene 6 Regulates Heme Oxygenase-1 Expression and Mitigates Hydrogen Peroxide-Induced Oxidative Stress in Lens Epithelial Cells.Curr Eye Res. 2022 Nov;47(11):1516-1524. doi: 10.1080/02713683.2022.2110266. Epub 2022 Sep 23. Curr Eye Res. 2022. PMID: 36149046
-
Biomarkers of oxidative stress and cataract. Novel drug delivery therapeutic strategies targeting telomere reduction and the expression of telomerase activity in the lens epithelial cells with N-acetylcarnosine lubricant eye drops: anti-cataract which helps to prevent and treat cataracts in the eyes of dogs and other animals.Curr Drug Deliv. 2014;11(1):24-61. doi: 10.2174/15672018113106660062. Curr Drug Deliv. 2014. PMID: 24783234 Review.
-
Redox Functions of Heme Oxygenase-1 and Biliverdin Reductase in Diabetes.Trends Endocrinol Metab. 2018 Feb;29(2):74-85. doi: 10.1016/j.tem.2017.11.005. Epub 2017 Dec 14. Trends Endocrinol Metab. 2018. PMID: 29249571 Review.
Cited by
-
Gold Nanoparticles Encapsulated Resveratrol as an Anti-Aging Agent to Delay Cataract Development.Pharmaceuticals (Basel). 2022 Dec 25;16(1):26. doi: 10.3390/ph16010026. Pharmaceuticals (Basel). 2022. PMID: 36678523 Free PMC article.
-
Endothelial senescence in vascular diseases: current understanding and future opportunities in senotherapeutics.Exp Mol Med. 2023 Jan;55(1):1-12. doi: 10.1038/s12276-022-00906-w. Epub 2023 Jan 4. Exp Mol Med. 2023. PMID: 36599934 Free PMC article. Review.
-
Klotho relieves H2O2-induced lens epithelial cell damage via suppression of NOX4.Int Ophthalmol. 2024 Nov 9;44(1):417. doi: 10.1007/s10792-024-03341-0. Int Ophthalmol. 2024. PMID: 39520585
-
HO-1-Mediated Autophagic Restoration Protects Lens Epithelial Cells Against Oxidative Stress and Cellular Senescence.Invest Ophthalmol Vis Sci. 2023 Dec 1;64(15):6. doi: 10.1167/iovs.64.15.6. Invest Ophthalmol Vis Sci. 2023. PMID: 38051262 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

