DL-3-N-Butylphthalide Promotes Cartilage Extracellular Matrix Synthesis and Inhibits Osteoarthritis Development by Regulating FoxO3a
- PMID: 35910845
- PMCID: PMC9329036
- DOI: 10.1155/2022/9468040
DL-3-N-Butylphthalide Promotes Cartilage Extracellular Matrix Synthesis and Inhibits Osteoarthritis Development by Regulating FoxO3a
Abstract
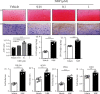
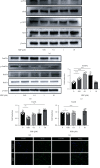
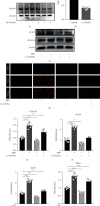
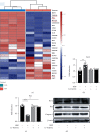
Osteoarthritis (OA) has been reported as a progressive disease in the elderly, primarily characterized by degenerated articular cartilage. There has been no satisfactory drug for the treatment of OA. DL-3-n-butylphthalide (NBP), a small molecule compound extracted from celery seeds, may have antiapoptotic, antioxidant, and anti-inflammatory activities in numerous studies. However, the effects of NBP on OA and its mechanisms have been rarely reported. In this study, the effect of NBP on OA in vitro and in vivo and its possible mechanism were investigated. The results showed that NBP injection into the knee joint inhibited osteoarthritis development in a rat model of osteoarthritis induced by DMM+ACLT. NBP could increase the expressions of extracellular matrix-related components (such as type II collagen, aggrecan, proteoglycan 4, and SRY-box 9) in human osteoarthritic chondrocytes and cartilage explants. Moreover, NBP promoted the expressions of SOD and CAT. NBP upregulated the expression of FoxO3a by inhibiting the PI3K/AKT pathway, which subsequently inhibited the apoptosis of human OA chondrocytes. In conclusion, NBP promotes cartilage extracellular matrix synthesis and inhibits osteoarthritis development and the underlying mechanism related to the activation of FoxO3a.
Copyright © 2022 Yaxin Zhang et al.
Conflict of interest statement
The authors declare no conflict of interest regarding the publication of this paper.
Figures





Similar articles
-
Downregulation of miR-34a Promotes Proliferation and Inhibits Apoptosis of Rat Osteoarthritic Cartilage Cells by Activating PI3K/Akt Pathway.Clin Interv Aging. 2020 Mar 16;15:373-385. doi: 10.2147/CIA.S241855. eCollection 2020. Clin Interv Aging. 2020. PMID: 32214804 Free PMC article.
-
Intra-articular injection of microRNA-140 (miRNA-140) alleviates osteoarthritis (OA) progression by modulating extracellular matrix (ECM) homeostasis in rats.Osteoarthritis Cartilage. 2017 Oct;25(10):1698-1707. doi: 10.1016/j.joca.2017.06.002. Epub 2017 Jun 21. Osteoarthritis Cartilage. 2017. PMID: 28647469
-
MiR-379-5p Promotes Chondrocyte Proliferation via Inhibition of PI3K/Akt Pathway by Targeting YBX1 in Osteoarthritis.Cartilage. 2022 Jan-Mar;13(1):19476035221074024. doi: 10.1177/19476035221074024. Cartilage. 2022. PMID: 35255737 Free PMC article.
-
[A new mechanism of action of chondroitin sulfates ACS4-ACS6 in osteoarthritic cartilage].Presse Med. 2002 Sep 14;31(29):1383-5. Presse Med. 2002. PMID: 12375394 Review. French.
-
Cartilage extracellular matrix-derived matrikines in osteoarthritis.Am J Physiol Cell Physiol. 2023 Feb 1;324(2):C377-C394. doi: 10.1152/ajpcell.00464.2022. Epub 2022 Dec 26. Am J Physiol Cell Physiol. 2023. PMID: 36571440 Review.
Cited by
-
TMF suppresses chondrocyte hypertrophy in osteoarthritic cartilage by mediating the FOXO3a/BMPER pathway.Exp Ther Med. 2024 May 15;28(1):283. doi: 10.3892/etm.2024.12571. eCollection 2024 Jul. Exp Ther Med. 2024. PMID: 38800044 Free PMC article.
-
Forkhead box O proteins in chondrocyte aging and diseases.J Orthop Translat. 2025 Aug 10;54:167-179. doi: 10.1016/j.jot.2025.07.011. eCollection 2025 Sep. J Orthop Translat. 2025. PMID: 40822516 Free PMC article. Review.
-
Guilu Erxian glue reduces endoplasmic reticulum stress-mediated apoptosis and restores the balance of extracellular matrix synthesis and degradation in chondrocytes by inhibiting the ATF6/GRP78/CHOP signaling pathway.Heliyon. 2024 Oct 31;10(24):e39987. doi: 10.1016/j.heliyon.2024.e39987. eCollection 2024 Dec 30. Heliyon. 2024. PMID: 39759286 Free PMC article.
-
Effect of 3D printing technology-assisted TKA on cartilage tissue in rabbit with knee osteoarthritis.Histol Histopathol. 2024 Dec;39(12):1631-1641. doi: 10.14670/HH-18-743. Epub 2024 Apr 5. Histol Histopathol. 2024. PMID: 38639204
-
The Roles of Forkhead Box O3a (FOXO3a) in Bone and Cartilage Diseases - A Narrative Review.Drug Des Devel Ther. 2025 Feb 27;19:1357-1375. doi: 10.2147/DDDT.S494841. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40034405 Free PMC article. Review.
References
-
- Musumeci G., Aiello F. C., Szychlinska M. A., Di Rosa M., Castrogiovanni P., Mobasheri A. Osteoarthritis in the XXIst century: risk factors and behaviours that influence disease onset and progression. International Journal of Molecular Sciences . 2015;16(12):6093–6112. doi: 10.3390/ijms16036093. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

