Salvianolic Acid A Protects against Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting Neutrophil NETosis
- PMID: 35910849
- PMCID: PMC9334034
- DOI: 10.1155/2022/7411824
Salvianolic Acid A Protects against Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting Neutrophil NETosis
Abstract
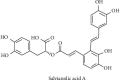
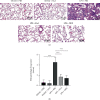
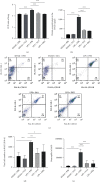
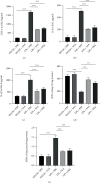
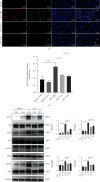
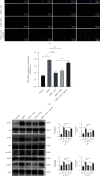
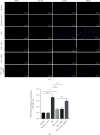
Salvianolic acid A (SAA) is one of bioactive polyphenol extracted from a Salvia miltiorrhiza (Danshen), which was widely used to treat cardiovascular disease in traditional Chinese medicine. SAA has been reported to be protective in cardiovascular disease and ischemia injury, with anti-inflammatory and antioxidative effect, but its role in acute lung injury (ALI) is still unknown. In this study, we sought to investigate the therapeutic effects of SAA in a murine model of lipopolysaccharide- (LPS-) induced ALI. The optimal dose of SAA was determined by comparing the attenuation of lung injury score after administration of SAA at three different doses (low, 5 mg/kg; medium, 10 mg/kg; and, high 15 mg/kg). Dexamethasone (DEX) was used as a positive control for SAA. Here, we showed that the therapeutic effect of SAA (10 mg/kg) against LPS-induced pathologic injury in the lungs was comparable to DEX. SAA and DEX attenuated the increased W/D ratio and the protein level, counts of total cells and neutrophils, and cytokine levels in the BALF of ALI mice similarly. The oxidative stress was also relieved by SAA and DEX according to the superoxide dismutase and malondialdehyde. NET level in the lungs was elevated in the injured lung while SAA and DEX reduced it significantly. LPS induced phosphorylation of Src, Raf, MEK, and ERK in the lungs, which was inhibited by SAA and DEX. NET level and phosphorylation level of Src/Raf/MEK/ERK pathway in the neutrophils from acute respiratory distress syndrome (ARDS) patients were also inhibited by SAA and DEX in vitro, but the YEEI peptide reversed the protective effect of SAA completely. The inhibition of NET release by SAA was also reversed by YEEI peptide in LPS-challenged neutrophils from healthy volunteers. Our data demonstrated that SAA ameliorated ALI via attenuating inflammation, oxidative stress, and neutrophil NETosis. The mechanism of such protective effect might involve the inhibition of Src activation.
Copyright © 2022 Qiang Liu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Three Ingredients of Safflower Alleviate Acute Lung Injury and Inhibit NET Release Induced by Lipopolysaccharide.Mediators Inflamm. 2020 Feb 29;2020:2720369. doi: 10.1155/2020/2720369. eCollection 2020. Mediators Inflamm. 2020. PMID: 32189992 Free PMC article.
-
β-Nitrostyrene derivatives attenuate LPS-mediated acute lung injury via the inhibition of neutrophil-platelet interactions and NET release.Am J Physiol Lung Cell Mol Physiol. 2018 Apr 1;314(4):L654-L669. doi: 10.1152/ajplung.00501.2016. Epub 2018 Jan 11. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 29351433
-
Icariside II alleviates lipopolysaccharide-induced acute lung injury by inhibiting lung epithelial inflammatory and immune responses mediated by neutrophil extracellular traps.Life Sci. 2024 Jun 1;346:122648. doi: 10.1016/j.lfs.2024.122648. Epub 2024 Apr 15. Life Sci. 2024. PMID: 38631668
-
The role of neutrophil extracellular traps in acute lung injury.Front Immunol. 2022 Jul 29;13:953195. doi: 10.3389/fimmu.2022.953195. eCollection 2022. Front Immunol. 2022. PMID: 35967320 Free PMC article. Review.
-
A Narrative Review: The Role of NETs in Acute Respiratory Distress Syndrome/Acute Lung Injury.Int J Mol Sci. 2024 Jan 25;25(3):1464. doi: 10.3390/ijms25031464. Int J Mol Sci. 2024. PMID: 38338744 Free PMC article. Review.
Cited by
-
Reactive Oxygen Species and Strategies for Antioxidant Intervention in Acute Respiratory Distress Syndrome.Antioxidants (Basel). 2023 Nov 18;12(11):2016. doi: 10.3390/antiox12112016. Antioxidants (Basel). 2023. PMID: 38001869 Free PMC article. Review.
-
Emerging Role of Neutrophil Extracellular Traps in Gastrointestinal Tumors: A Narrative Review.Int J Mol Sci. 2022 Dec 25;24(1):334. doi: 10.3390/ijms24010334. Int J Mol Sci. 2022. PMID: 36613779 Free PMC article. Review.
-
Advances in acute respiratory distress syndrome: focusing on heterogeneity, pathophysiology, and therapeutic strategies.Signal Transduct Target Ther. 2025 Mar 7;10(1):75. doi: 10.1038/s41392-025-02127-9. Signal Transduct Target Ther. 2025. PMID: 40050633 Free PMC article. Review.
-
Neutrophil extracellular traps in homeostasis and disease.Signal Transduct Target Ther. 2024 Sep 20;9(1):235. doi: 10.1038/s41392-024-01933-x. Signal Transduct Target Ther. 2024. PMID: 39300084 Free PMC article. Review.
-
Neutrophil-specific ORAI1 Calcium Channel Inhibition Reduces Pancreatitis-associated Acute Lung Injury.Function (Oxf). 2023 Oct 23;5(1):zqad061. doi: 10.1093/function/zqad061. eCollection 2024. Function (Oxf). 2023. PMID: 38020066 Free PMC article.
References
-
- Yoshida T., Fujino Y., Amato M. B., Kavanagh B. P. Fifty years of research in ARDS. Spontaneous breathing during mechanical ventilation. Risks, mechanisms, and management. American Journal of Respiratory and Critical Care Medicine . 2017;195(8):985–992. doi: 10.1164/rccm.201604-0748CP. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

