Basophil Activation Test as a Biomarker for Taxanes Anaphylaxis
- PMID: 35910859
- PMCID: PMC9328177
- DOI: 10.3389/falgy.2022.787749
Basophil Activation Test as a Biomarker for Taxanes Anaphylaxis
Abstract
Introduction: Taxanes are widely used chemotherapy agents, and their administration, despite premedication, is associated with hypersensitivity reactions (HR) in up to 9% of patients, 1% of which are severe. The mechanisms of these reactions are not fully understood. Finding biomarkers for early diagnosis and better understanding the underlying mechanisms of these reactions are key to defining the best treatment strategy for patients.
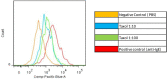
Methods: The purpose of this study was to evaluate the effectiveness of the basophil activation test (BAT) to diagnose patients with anaphylactic reactions to taxanes. Patients with anaphylaxis to taxane compounds (n = 15) were assessed through clinical history, skin testing (when possible), and BAT. BAT was performed immediately before rapid drug desensitization or before skin testing using anti-CD123 conjugated (APC-Biolegend), anti-HLADR conjugated (FITC-Biolegend) to gate Basophils and anti-CD63 conjugated (PE-Biolegend), and anti-CD203c conjugated (BV-Biolegend) to assess CD203c and CD63 expression on basophils under taxane stimulation. BAT was also performed in eight healthy volunteers.
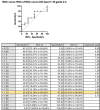
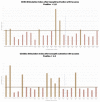
Results: BAT was positive for CD203c in eight out of 15 patients and for CD63 in four out of 15 patients and in two out of eight controls. The sensitivity for CD203c was 53%, the specificity was 87%, and the area under the curve was 0.66 (p = 0.19%). For CD63, these rates were 33%, 87%, and 0.6 (p = 0.4). In a subgroup analysis of patients with positive skin tests (11 patients), CD203c was positive in six patients (sensitivity of 54.5% and specificity of 87.5%), and CD63 was positive in five patients (sensitivity of 45% and specificity of 75%).
Conclusions: BAT as a diagnostic tool for immediate hypersensitivity reactions to taxanes may be relevant in patients with selected phenotypes and endotypes, especially those with severe reactions or when the diagnosis cannot be established by the skin test. Increased expression of CD203c was more frequent than of CD63 in patients with positive results, and the sensitivity of this biomarker was higher in patient sub-group with positive skin tests, i.e., patients with IgE-mediated endotypes.
Keywords: anaphylaxis; basophil activation test (BAT); chemotherapy; immediate hypersensitivity reaction; taxanes agents.
Copyright © 2022 Campos, Giavina-Bianchi, Acharya, Lynch, Kalil and Castells.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Basophil Activation Test is a Relevant Biomarker of the Outcome of Rapid Desensitization in Platinum Compounds-Allergy.J Allergy Clin Immunol Pract. 2017 May-Jun;5(3):728-736. doi: 10.1016/j.jaip.2016.11.006. Epub 2016 Dec 27. J Allergy Clin Immunol Pract. 2017. PMID: 28034549
-
Diagnosis of immediate reactions to amoxicillin: Comparison of basophil activation markers CD63 and CD203c in a prospective study.Allergy. 2023 Oct;78(10):2745-2755. doi: 10.1111/all.15610. Epub 2022 Dec 17. Allergy. 2023. PMID: 36478407
-
Comparison of two basophil activation markers CD63 and CD203c in the diagnosis of amoxicillin allergy.Clin Exp Allergy. 2008 Jun;38(6):921-8. doi: 10.1111/j.1365-2222.2008.02960.x. Epub 2008 Mar 6. Clin Exp Allergy. 2008. PMID: 18331364 Clinical Trial.
-
Basophil Activation Experiments in Immediate Drug Hypersensitivity: More Than a Diagnostic Aid.Methods Mol Biol. 2020;2163:197-211. doi: 10.1007/978-1-0716-0696-4_16. Methods Mol Biol. 2020. PMID: 32766977 Review.
-
In Vitro Diagnosis of Immediate Drug Hypersensitivity Anno 2017: Potentials and Limitations.Drugs R D. 2017 Jun;17(2):265-278. doi: 10.1007/s40268-017-0176-x. Drugs R D. 2017. PMID: 28258478 Free PMC article. Review.
Cited by
-
Basophil Activation Test: Bridging Allergy and Oncology for Diagnostic, Therapeutic and Prognostic Applications in AllergoOncology: An EAACI Position Paper.Allergy. 2025 Aug;80(8):2097-2112. doi: 10.1111/all.16607. Epub 2025 Jun 12. Allergy. 2025. PMID: 40503572 Free PMC article. Review.
-
A Modified Basophil Activation Test for the Clinical Management of Immediate Hypersensitivity Reactions to Paclitaxel: A Proof-of-Concept Study.Cancers (Basel). 2023 Dec 13;15(24):5818. doi: 10.3390/cancers15245818. Cancers (Basel). 2023. PMID: 38136365 Free PMC article.
-
Basophil activation test is a complementary tool in the diagnosis of immediate reactions to platinum salts and taxanes.Allergy. 2025 Jan;80(1):271-286. doi: 10.1111/all.16296. Epub 2024 Aug 31. Allergy. 2025. PMID: 39215539 Free PMC article.
References
-
- INCA B. Estimate/2020 - Cancer Incidence in Brazil. COORDENAÇÃO DE ENSINO. Serviço de Educação e Informação Técnico-Cient#x000ED;fica ed. Rio de Janeiro: Brazil; (2019). p. 120.
LinkOut - more resources
Full Text Sources
Miscellaneous

