β-arrestin-1 and β-arrestin-2 Restrain MRGPRX2-Triggered Degranulation and ERK1/2 Activation in Human Skin Mast Cells
- PMID: 35910860
- PMCID: PMC9337275
- DOI: 10.3389/falgy.2022.930233
β-arrestin-1 and β-arrestin-2 Restrain MRGPRX2-Triggered Degranulation and ERK1/2 Activation in Human Skin Mast Cells
Abstract
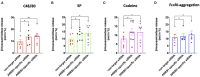
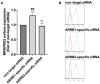
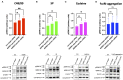
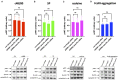
As a novel receptor that efficiently elicits degranulation upon binding to one of its numerous ligands, MRGPRX2 has moved to the center of attention in mast cell (MC) research. Indeed, MRGPRX2 is believed to be a major component of pseudo-allergic reactions to drugs and of neuropeptide-elicited MC activation in skin diseases alike. MRGPRX2 signals via G proteins which organize downstream events ultimately leading to granule discharge. Skin MCs require both PI3K and ERK1/2 cascades for efficient exocytosis. β-arrestins act as opponents of G proteins and lead to signal termination with or without subsequent internalization. We recently demonstrated that ligand-induced internalization of MRGPRX2 requires the action of β-arrestin-1, but not of β-arrestin-2. Here, by using RNA interference, we find that both isoforms counter skin MC degranulation elicited by three MRGPRX2 agonists but not by FcεRI-aggregation. Analyzing whether this occurs through MRGPRX2 stabilization under β-arrestin attenuation, we find that reduction of β-arrestin-1 indeed leads to increased MRGPRX2 abundance, while this is not observed for β-arrestin-2. This led us speculate that β-arrestin-2 is involved in signal termination without cellular uptake of MRGPRX2. This was indeed found to be the case, whereby interference with β-arrestin-2 has an even stronger positive effect on ERK1/2 phosphorylation compared to β-arrestin-1 perturbation. Neither β-arrestin-1 nor β-arrestin-2 had an impact on AKT phosphorylation nor affected signaling via the canonical FcεRI-dependent route. We conclude that in skin MCs, β-arrestin-2 is chiefly involved in signal termination, whereas β-arrestin-1 exerts its effects by controlling MRGPRX2 abundance.
Keywords: ERK1/2; MRGPRX2; degranulation; mast cells; signal transduction; skin; β-arrestin.
Copyright © 2022 Wang, Li, Bal, Franke, Zuberbier and Babina.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
MRGPRX2-Mediated Degranulation of Human Skin Mast Cells Requires the Operation of Gαi, Gαq, Ca++ Channels, ERK1/2 and PI3K-Interconnection between Early and Late Signaling.Cells. 2022 Mar 10;11(6):953. doi: 10.3390/cells11060953. Cells. 2022. PMID: 35326404 Free PMC article.
-
Thymic Stromal Lymphopoietin Promotes MRGPRX2-Triggered Degranulation of Skin Mast Cells in a STAT5-Dependent Manner with Further Support from JNK.Cells. 2021 Jan 8;10(1):102. doi: 10.3390/cells10010102. Cells. 2021. PMID: 33429916 Free PMC article.
-
MRGPRX2 Is the Codeine Receptor of Human Skin Mast Cells: Desensitization through β-Arrestin and Lack of Correlation with the FcεRI Pathway.J Invest Dermatol. 2021 May;141(5):1286-1296.e4. doi: 10.1016/j.jid.2020.09.017. Epub 2020 Oct 13. J Invest Dermatol. 2021. PMID: 33058860 Free PMC article.
-
MRGPRX2, drug pseudoallergies, inflammatory diseases, mechanisms and distinguishing MRGPRX2- and IgE/FcεRI-mediated events.Br J Clin Pharmacol. 2023 Nov;89(11):3232-3246. doi: 10.1111/bcp.15845. Epub 2023 Aug 8. Br J Clin Pharmacol. 2023. PMID: 37430437 Review.
-
MRGPRX2 signals its importance in cutaneous mast cell biology: Does MRGPRX2 connect mast cells and atopic dermatitis?Exp Dermatol. 2020 Nov;29(11):1104-1111. doi: 10.1111/exd.14182. Epub 2020 Sep 17. Exp Dermatol. 2020. PMID: 32866307 Review.
Cited by
-
ClickArr: a novel, high-throughput assay for evaluating β-arrestin isoform recruitment.Front Pharmacol. 2023 Nov 7;14:1295518. doi: 10.3389/fphar.2023.1295518. eCollection 2023. Front Pharmacol. 2023. PMID: 38027002 Free PMC article.
-
Synergism between IL-33 and MRGPRX2/FcεRI Is Primarily Due to the Complementation of Signaling Modules, and Only Modestly Supplemented by Prolonged Activation of Selected Kinases.Cells. 2023 Nov 24;12(23):2700. doi: 10.3390/cells12232700. Cells. 2023. PMID: 38067128 Free PMC article.
-
Potential Role of Moesin in Regulating Mast Cell Secretion.Int J Mol Sci. 2023 Jul 28;24(15):12081. doi: 10.3390/ijms241512081. Int J Mol Sci. 2023. PMID: 37569454 Free PMC article. Review.
-
CREB Is Indispensable to KIT Function in Human Skin Mast Cells-A Positive Feedback Loop between CREB and KIT Orchestrates Skin Mast Cell Fate.Cells. 2023 Dec 24;13(1):42. doi: 10.3390/cells13010042. Cells. 2023. PMID: 38201246 Free PMC article.
-
CREB Is Activated by the SCF/KIT Axis in a Partially ERK-Dependent Manner and Orchestrates Survival and the Induction of Immediate Early Genes in Human Skin Mast Cells.Int J Mol Sci. 2023 Feb 18;24(4):4135. doi: 10.3390/ijms24044135. Int J Mol Sci. 2023. PMID: 36835547 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

