The Vaccine Efficacy Against the SARS-CoV-2 Omicron: A Systemic Review and Meta-Analysis
- PMID: 35910897
- PMCID: PMC9326247
- DOI: 10.3389/fpubh.2022.940956
The Vaccine Efficacy Against the SARS-CoV-2 Omicron: A Systemic Review and Meta-Analysis
Abstract
Background: COVID-19 is a respiratory illness caused by SARS-CoV-2. The most recent variant is Omicron (line B.1.1.529), which was first identified in South Africa in November 2021. The concern with this variant is the ineffectiveness of vaccines currently available. We aim to systematically evaluate the effectiveness of the currently available COVID-19 vaccines and boosters for the Omicron variant.
Methods: We searched the PubMed, Embase, the Cochrane Library and Web of Science databases from inception to June 5th, 2022. Studies that examined the effectiveness of SARS-CoV-2 vaccines against the Omicron variant infection were included. Random-effects model was used to estimate the pooled vaccine effectiveness against the Omicron variant.
Results: A total of 13 studies were included to evaluate the effectiveness of the vaccine against the Omicron variant, and 11 studies were included to compare the effectiveness between the two-dose and three-dose (booster) vaccinations. Full vaccination (two-dose with or without booster) showed a protective effect against the Omicron variant compared to no vaccination (OR = 0.62, 95% CI: 0.56-0.69), while the effectiveness decreased significantly over 6 months after the last dose. The two-dose vaccination plus booster provided better protection against the Omicron variant compared to the two-dose vaccination without booster (OR = 0.60, 95% CI: 0.52-0.68). Additional analysis was performed for the most commonly used vaccines in the United Staes: BNT162b2(Pfizer) (OR = 0.65, 95% CI: 0.52-0.82) and mRNA-1273(Moderna) (OR = 0.67, 95% CI: 0.58-0.88) vaccines in the US, which showed similar effectiveness compared to no vaccination.
Conclusions: The full dose of SARS-CoV-2 vaccination effectively reduces infection from the SARS-CoV-2 Omicron variant; however, the effectiveness wanes over time. The booster vaccine provides additional protection against the Omicron variant.
Keywords: Omicron variant; booster; infection; meta-analysis; vaccination.
Copyright © 2022 Zou, Huang, Jiang, Guo and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
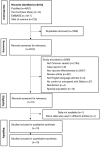

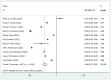
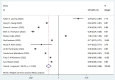
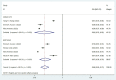
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

