ADSCs Combined with Melatonin Promote Peripheral Nerve Regeneration through Autophagy
- PMID: 35910940
- PMCID: PMC9329031
- DOI: 10.1155/2022/5861553
ADSCs Combined with Melatonin Promote Peripheral Nerve Regeneration through Autophagy
Abstract
Background: In the early stage of nerve injury, damaged tissue is cleared by autophagy. ADSCs can promote nerve axon regeneration. However, the microenvironment of the injury was changed, and ADSCs are easily apoptotic after transplantation. Mel plays a role in the apoptosis, proliferation, and differentiation of ADSCs. Therefore, we investigated whether Mel combined with ADSCs promoted peripheral nerve regeneration by enhancing early autophagy of injured nerves.
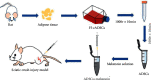
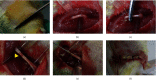
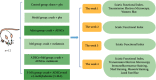
Materials and methods: SD rats were randomly split into the control group, model group, Mel group, ADSCs group, ADSCs + Mel group, and 3-MA group. On day 7, autophagy was observed and gait was detected on days 7, 14, 21, and 28. On the 28th day, the sciatic nerve of rats' renewal was detected.
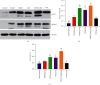
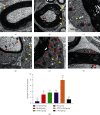
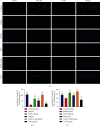
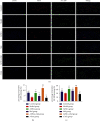
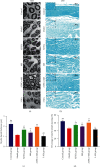
Results: After 1 w, compare with the model group, the number of autophagosomes and lysosomes and the expressions of protein of LC3-II/LC3-I and Beclin-1 in the ADSCs + Mel group were prominently increased, while the 3-MA group was significantly decreased. After 4 w, the function of the sciatic nerve in ADSCs + Mel was similar to that in the control group. Compared with the model group, the ADSCs + Mel group significantly increased myelin regeneration and the number of motor neurons and reduced gastrocnemius atrophy.
Conclusions: It was confirmed that ADSCs combined with Mel could promote sciatic nerve regeneration in rats by changing the early autophagy activity of the injured sciatic nerve.
Copyright © 2022 Ziqiang Zhang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Induction of Autophagy and Its Role in Peripheral Nerve Regeneration after Peripheral Nerve Injury.Int J Mol Sci. 2023 Nov 11;24(22):16219. doi: 10.3390/ijms242216219. Int J Mol Sci. 2023. PMID: 38003409 Free PMC article. Review.
-
Effects of adipose derived stem cells pretreated with resveratrol on sciatic nerve regeneration in rats.Sci Rep. 2023 Apr 10;13(1):5812. doi: 10.1038/s41598-023-32906-9. Sci Rep. 2023. PMID: 37037844 Free PMC article.
-
Autophagy Promotes Peripheral Nerve Regeneration and Motor Recovery Following Sciatic Nerve Crush Injury in Rats.J Mol Neurosci. 2016 Apr;58(4):416-23. doi: 10.1007/s12031-015-0672-9. Epub 2016 Jan 7. J Mol Neurosci. 2016. PMID: 26738732 Free PMC article.
-
[Effects of adipose-derived mesenchymal stem cells over-expressing glial cell line-derived neurotrophic factor on electrically injured sciatic nerve of rats].Zhonghua Shao Shang Za Zhi. 2015 Jun;31(3):199-204. Zhonghua Shao Shang Za Zhi. 2015. PMID: 26564567 Chinese.
-
Bone marrow-derived mesenchymal stem cells versus adipose-derived mesenchymal stem cells for peripheral nerve regeneration.Neural Regen Res. 2018 Jan;13(1):100-104. doi: 10.4103/1673-5374.224378. Neural Regen Res. 2018. PMID: 29451213 Free PMC article.
Cited by
-
Melatonin signalling in Schwann cells during neuroregeneration.Front Cell Dev Biol. 2022 Oct 10;10:999322. doi: 10.3389/fcell.2022.999322. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36299487 Free PMC article. Review.
-
Electroacupuncture Promotes Functional Recovery after Facial Nerve Injury in Rats by Regulating Autophagy via GDNF and PI3K/mTOR Signaling Pathway.Chin J Integr Med. 2024 Mar;30(3):251-259. doi: 10.1007/s11655-023-3610-7. Epub 2024 Jan 12. Chin J Integr Med. 2024. PMID: 38212498
-
[Role and mechanism of Prussian blue nanoparticles in the apoptosis of mouse adipose-derived mesenchymal stem cells treated with hydrogen peroxide].Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2025 May 20;41(5):481-490. doi: 10.3760/cma.j.cn501225-20240525-00197. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2025. PMID: 40419362 Free PMC article. Chinese.
-
Induction of Autophagy and Its Role in Peripheral Nerve Regeneration after Peripheral Nerve Injury.Int J Mol Sci. 2023 Nov 11;24(22):16219. doi: 10.3390/ijms242216219. Int J Mol Sci. 2023. PMID: 38003409 Free PMC article. Review.
-
Biological nerve conduit model with de-epithelialized human amniotic membrane and adipose-derived mesenchymal stem cell sheet for repair of peripheral nerve defects.Cell Tissue Res. 2023 Mar;391(3):505-522. doi: 10.1007/s00441-022-03732-8. Epub 2022 Dec 23. Cell Tissue Res. 2023. PMID: 36562866
References
LinkOut - more resources
Full Text Sources

