A Biologically Inspired Neural Network Model to Gain Insight Into the Mechanisms of Post-Traumatic Stress Disorder and Eye Movement Desensitization and Reprocessing Therapy
- PMID: 35911047
- PMCID: PMC9326218
- DOI: 10.3389/fpsyg.2022.944838
A Biologically Inspired Neural Network Model to Gain Insight Into the Mechanisms of Post-Traumatic Stress Disorder and Eye Movement Desensitization and Reprocessing Therapy
Abstract
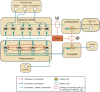
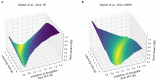
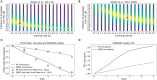
Eye movement desensitization and reprocessing (EMDR) therapy is a well-established therapeutic method to treat post-traumatic stress disorder (PTSD). However, how EMDR exerts its therapeutic action has been studied in many types of research but still needs to be completely understood. This is in part due to limited knowledge of the neurobiological mechanisms underlying EMDR, and in part to our incomplete understanding of PTSD. In order to model PTSD, we used a biologically inspired computational model based on firing rate units, encompassing the cortex, hippocampus, and amygdala. Through the modulation of its parameters, we fitted real data from patients treated with EMDR or classical exposure therapy. This allowed us to gain insights into PTSD mechanisms and to investigate how EMDR achieves trauma remission.
Keywords: amygdala; computational modeling; eye movement desensitization and reprocessing therapy; post-traumatic stress disorder; prolonged exposure.
Copyright © 2022 Mattera, Cavallo, Granato, Baldassarre and Pagani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
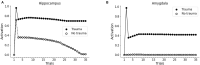





References
LinkOut - more resources
Full Text Sources

