Hypercatabolism and Anti-catabolic Therapies in the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome
- PMID: 35911117
- PMCID: PMC9326442
- DOI: 10.3389/fnut.2022.941097
Hypercatabolism and Anti-catabolic Therapies in the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome
Abstract
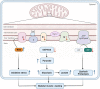
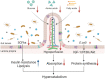
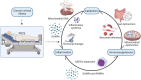
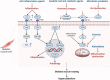
Owing to the development of intensive care units, many patients survive their initial insults but progress to chronic critical illness (CCI). Patients with CCI are characterized by prolonged hospitalization, poor outcomes, and significant long-term mortality. Some of these patients get into a state of persistent low-grade inflammation, suppressed immunity, and ongoing catabolism, which was defined as persistent inflammation, immunosuppression, and catabolism syndrome (PICS) in 2012. Over the past few years, some progress has been made in the treatment of PICS. However, most of the existing studies are about the role of persistent inflammation and suppressed immunity in PICS. As one of the hallmarks of PICS, hypercatabolism has received little research attention. In this review, we explore the potential pathophysiological changes and molecular mechanisms of hypercatabolism and its role in PICS. In addition, we summarize current therapies for improving the hypercatabolic status and recommendations for patients with PICS.
Keywords: and catabolism syndrome; anti-catabolic therapy; chronic critical illness; gut dysfunction; hypercatabolism; immunosuppression; mitochondrial dysfunction; persistent inflammation.
Copyright © 2022 Zhang, Luo, Miao and Zhong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Persistent inflammation, immunosuppression, and catabolism syndrome (PICS): a review of definitions, potential therapies, and research priorities.Br J Anaesth. 2024 Mar;132(3):507-518. doi: 10.1016/j.bja.2023.11.052. Epub 2024 Jan 4. Br J Anaesth. 2024. PMID: 38177003 Free PMC article. Review.
-
Inflammatory Response and Anti-Inflammatory Treatment in Persistent Inflammation-Immunosuppression-Catabolism Syndrome (PICS).J Inflamm Res. 2025 Feb 14;18:2267-2281. doi: 10.2147/JIR.S504694. eCollection 2025. J Inflamm Res. 2025. PMID: 39968098 Free PMC article. Review.
-
Chronic Critical Illness and PICS Nutritional Strategies.J Clin Med. 2021 May 25;10(11):2294. doi: 10.3390/jcm10112294. J Clin Med. 2021. PMID: 34070395 Free PMC article. Review.
-
Innate Immunity in the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome and Its Implications for Therapy.Front Immunol. 2018 Apr 4;9:595. doi: 10.3389/fimmu.2018.00595. eCollection 2018. Front Immunol. 2018. PMID: 29670613 Free PMC article. Review.
-
[Persistent inflammation immunosuppression catabolism syndrome: a special type of chronic critical illness].Zhonghua Wei Chang Wai Ke Za Zhi. 2016 Jul;19(7):734-6. Zhonghua Wei Chang Wai Ke Za Zhi. 2016. PMID: 27452746 Chinese.
Cited by
-
Prognostic value of preoperative nutritional status for postoperative moderate to severe acute kidney injury among older patients undergoing coronary artery bypass graft surgery: a retrospective study based on the MIMIC-IV database.Ren Fail. 2024 Dec;46(2):2429683. doi: 10.1080/0886022X.2024.2429683. Epub 2024 Dec 1. Ren Fail. 2024. PMID: 39618077 Free PMC article.
-
Persistent inflammation, immunosuppression, and catabolism syndrome (PICS): a review of definitions, potential therapies, and research priorities.Br J Anaesth. 2024 Mar;132(3):507-518. doi: 10.1016/j.bja.2023.11.052. Epub 2024 Jan 4. Br J Anaesth. 2024. PMID: 38177003 Free PMC article. Review.
-
Impact of Persistent Inflammation, Immunosuppression, and Catabolism Syndrome during Intensive Care Admission on Each Post-Intensive Care Syndrome Component in a PICS Clinic.J Clin Med. 2023 Aug 21;12(16):5427. doi: 10.3390/jcm12165427. J Clin Med. 2023. PMID: 37629468 Free PMC article.
-
Addressing Inflammaging and Disease-Related Malnutrition: Adequacy of Oral Nutritional Supplements in Clinical Care.Nutrients. 2024 Nov 29;16(23):4141. doi: 10.3390/nu16234141. Nutrients. 2024. PMID: 39683535 Free PMC article. Review.
-
Navigating Nutritional Strategies: Permissive Underfeeding in Critically Ill Patients.Cureus. 2024 Apr 11;16(4):e58083. doi: 10.7759/cureus.58083. eCollection 2024 Apr. Cureus. 2024. PMID: 38741818 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

