Antimicrobial Alkaloids from Marine-Derived Fungi as Drug Leads versus COVID-19 Infection: A Computational Approach to Explore their Anti-COVID-19 Activity and ADMET Properties
- PMID: 35911157
- PMCID: PMC9325633
- DOI: 10.1155/2022/5403757
Antimicrobial Alkaloids from Marine-Derived Fungi as Drug Leads versus COVID-19 Infection: A Computational Approach to Explore their Anti-COVID-19 Activity and ADMET Properties
Abstract
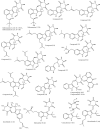
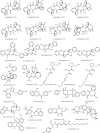
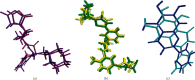
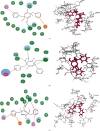
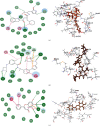
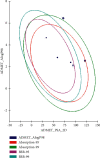
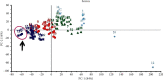
Therapeutic strategies based upon enzyme inhibition have recently gained higher attention in treating hazardous ailments. Herein, the potential use of seventy-two antimicrobial alkaloids isolated from marine-derived fungi to fight COVID-19 infection via inhibition of SARS-CoV-2 lethal virus was performed using in silico analyses. Molecular modelling was performed to assess their enzyme inhibitory potential on the main protease SARS-CoV-2 MPro, 3-chymotrypsin-like protease SARS-CoV-2 3CLpro, and papain-like protease SARS-CoV-2 PLpro using Discovery Studio 4.5. Validation of the docking experiments was done by determination of RMSD (root mean square deviation) after redocking the superimposition of the cocrystalized ligands. Results showed that gymnastatin Z (72) showed the best fitting score in SARS-CoV-2 MPro and SARS-CoV-2 3CLpr active sites with ∆G equal -34.15 and -34.28 Kcal/mol, respectively. Meanwhile, scalusamide C (62) displayed the highest fitting within SARS-CoV-2 PLpro active sites (∆G = -26.91 Kcal/mol) followed by eutypellazine M (57). ADMET/TOPKAT prediction displayed that eutypellazine M and scalusamide C showed better pharmacokinetic and pharmacodynamic properties. Gymnastatin Z is safer showing better toxicity criteria and higher rat oral LD50 and rat chronic LOAEL (lowest observed adverse effect level). Chemometric analysis using principle component analysis (PCA) based on the binding energies observed for the compounds with respect to the three tested enzymes revealed the clustering of the compounds into different clusters. Eutypellazine M, scalusamide C, and gymnastatin Z appear in one cluster due to their closeness in activity. Thus, these compounds could serve as promising SARS-CoV-2 enzymes inhibitors that could help in alleviation of COVID-19 infection. Further investigations are recommended to confirm the results of molecular modelling.
Copyright © 2022 Sherouk Hussein Sweilam et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





Similar articles
-
The Role of Cannabis sativa L. as a Source of Cannabinoids against Coronavirus 2 (SARS-CoV-2): An In Silico Study to Evaluate Their Activities and ADMET Properties.Molecules. 2022 Apr 27;27(9):2797. doi: 10.3390/molecules27092797. Molecules. 2022. PMID: 35566148 Free PMC article.
-
Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants - Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi) - a molecular docking study.J Biomol Struct Dyn. 2022 Jan;40(1):190-203. doi: 10.1080/07391102.2020.1810778. Epub 2020 Aug 27. J Biomol Struct Dyn. 2022. PMID: 32851919 Free PMC article.
-
Potential inhibitors of coronavirus 3-chymotrypsin-like protease (3CLpro): an in silico screening of alkaloids and terpenoids from African medicinal plants.J Biomol Struct Dyn. 2021 Jun;39(9):3396-3408. doi: 10.1080/07391102.2020.1764868. Epub 2020 May 18. J Biomol Struct Dyn. 2021. PMID: 32367767 Free PMC article.
-
Computational Evidences of Phytochemical Mediated Disruption of PLpro Driven Replication of SARS-CoV-2: A Therapeutic Approach against COVID-19.Curr Pharm Biotechnol. 2021;22(10):1350-1359. doi: 10.2174/1389201021999201110204116. Curr Pharm Biotechnol. 2021. PMID: 33176643
-
Potential inhibitors of SARS-CoV-2 (COVID 19) proteases PLpro and Mpro/ 3CLpro: molecular docking and simulation studies of three pertinent medicinal plant natural components.Curr Res Pharmacol Drug Discov. 2021;2:100038. doi: 10.1016/j.crphar.2021.100038. Epub 2021 Jun 5. Curr Res Pharmacol Drug Discov. 2021. PMID: 34870149 Free PMC article.
Cited by
-
Predicting the Anti-SARS-CoV-2 Potential of Isoquinoline Alkaloids from Brazilian Siparunaceae Species Using Chemometric Tools.Int J Mol Sci. 2025 Jan 13;26(2):633. doi: 10.3390/ijms26020633. Int J Mol Sci. 2025. PMID: 39859347 Free PMC article.
-
Metabolic Profiling and Investigation of the Modulatory Effect of Fagonia cretica L. Aerial Parts on Hepatic CYP3A4 and UGT2B7 Enzymes in Streptozotocin-Induced Diabetic Model.Antioxidants (Basel). 2023 Jan 3;12(1):119. doi: 10.3390/antiox12010119. Antioxidants (Basel). 2023. PMID: 36670981 Free PMC article.
-
Screening for Neuroprotective and Rapid Antidepressant-like Effects of 20 Essential Oils.Biomedicines. 2023 Apr 23;11(5):1248. doi: 10.3390/biomedicines11051248. Biomedicines. 2023. PMID: 37238920 Free PMC article.
-
Marine Aspergillus: A Treasure Trove of Antimicrobial Compounds.Mar Drugs. 2023 Apr 28;21(5):277. doi: 10.3390/md21050277. Mar Drugs. 2023. PMID: 37233471 Free PMC article. Review.
References
-
- Abadines I. B., Le K., Newman D. J., Glaser K. B., Mayer A. M. The marine pharmacology and pharmaceuticals pipeline in 2018. FASEB Journal . 2019;33
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

