Urinary CD8+HLA-DR+ T Cell Abundance Non-invasively Predicts Kidney Transplant Rejection
- PMID: 35911418
- PMCID: PMC9334669
- DOI: 10.3389/fmed.2022.928516
Urinary CD8+HLA-DR+ T Cell Abundance Non-invasively Predicts Kidney Transplant Rejection
Abstract
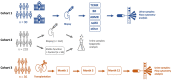
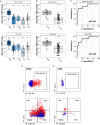
Early detection of kidney transplant (KT) rejection remains a challenge in patient care. Non-invasive biomarkers hold high potential to detect rejection, adjust immunosuppression, and monitor KT patients. So far, no approach has fully satisfied requirements to innovate routine monitoring of KT patients. In this two-center study we analyzed a total of 380 urine samples. T cells and tubular epithelial cells were quantified in KT patients with graft deterioration using flow cytometry. Epigenetic urine cell quantification was used to confirm flow cytometric results. Moreover, a cohort of KT patients was followed up during the first year after transplantation, tracking cell subsets over time. Abundance of urinary cell counts differed in patients with and without rejection. Most strikingly, various T cell subsets were enriched in patients with T cell-mediated rejection (TCMR) compared to patients without TCMR. Among T cell subsets, CD8+HLA-DR+ T cells were most distinctive (AUC = 0.91, Spec.: 95.9%, Sens.: 76.5%). Epigenetic analysis confirmed T cell and tubular epithelial cell quantities as determined by flow cytometry. Urinary T cell abundance in new KT patients decreased during their first year after transplantation. In conclusion urinary T cells reflect intrarenal inflammation in TCMR. T cell subsets yield high potential to monitor KT patients and detect rejection. Hereby we present a promising biomarker to non-invasively diagnose TCMR.
Keywords: CD8+HLA-DR+; T cell; allograft acute rejection; biomarker; kidney; transplantation; tubular epithelial cell; urine.
Copyright © 2022 Grothgar, Goerlich, Samans, Skopnik, Metzke, Klocke, Prskalo, Freund, Wagner, Duerr, Matz, Olek, Budde, Paliege and Enghard.
Conflict of interest statement
BS and SO are employed by Precision for Medicine GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- COPE. Official Journal of the international Society of Nephrology KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Available online at: www.publicationethics.org (accessed April 23, 2022).
LinkOut - more resources
Full Text Sources
Research Materials

