Past, present and future in low-risk myelodysplastic syndrome
- PMID: 35911422
- PMCID: PMC9334722
- DOI: 10.3389/fmed.2022.967900
Past, present and future in low-risk myelodysplastic syndrome
Abstract
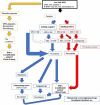
Myelodysplastic syndromes (MDS) is a heterogeneous group of disorders characterized by increased risk of acute myeloid leukemia transformation and cytopenia. The prognosis of MDS patients can be evaluated with various scoring systems, the most commonly used are IPSS (International Prognostic Scoring System), revised-IPSS, and WPSS (WHO classification-based prognostic scoring system). MDS treatment is decided according to the risk classification. The goal of treatment in low-risk MDS is to improve cytopenia, reduce transfusion needs, improve quality of life, prolong overall survival, and maybe reduce the risk of progression to leukemia. In the near future, combining both genomics-based, ex vivo functional based and molecular stratification analysis will lead the way to a personalized and targeted approach.
Keywords: anemia; low risk; myelodysplastic syndrome; thrombocytopenia; treatment.
Copyright © 2022 Toprak.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

