Binding of Dual-Function Hybridized Metal - Organic Capsules to Enzymes for Cascade Catalysis
- PMID: 35911460
- PMCID: PMC9327082
- DOI: 10.1021/jacsau.2c00322
Binding of Dual-Function Hybridized Metal - Organic Capsules to Enzymes for Cascade Catalysis
Abstract
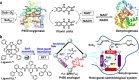
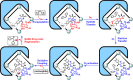
The combination of chemo- and biocatalysis for multistep syntheses provides attractive advantages in terms of evolvability, promiscuity, and sustainability striving for desirable catalytic performance. Through the encapsulation of flavin analogues by both NADH and heme mimics codecorated heteroleptic metal-organic capsules, herein, we report a progressive host-guest strategy to imitate cytochrome P450s catalysis for cascade oxidative coupling catalysis. Besides the construction of stable dual-function metal-organic capsules and the modification of cofactor-decorated capsules at the domain of enzymes, this supramolecular strategy involves multistage directional electron flow, affording reactive ferric peroxide species for inducing oxygenation. Under light irradiation, the metal-organic capsule selectively converts stilbene to oxidative coupling products (including 2-oxo-1,2-diphenylethyl formate, 2-alkoxy-1,2-diphenylethanone) in tandem with enzymatic reactions respectively, at the domain of natural enzymes. The ingenious combination of capsules and enzymes with the in situ-regenerated capsule-loaded NADH cofactor promises non-native coupling reactions by forming regional cooperation and division. This abiotic-biotic conjugated host-guest strategy is conducive to the de novo creation of multifunctional components approaching active enzymatic sites for reinforced matter and energy transporting, demonstrating a key role of multicomponent supramolecular catalysts for one-pot integrated catalytic conversions.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
A host-guest approach to combining enzymatic and artificial catalysis for catalyzing biomimetic monooxygenation.Nat Commun. 2020 Jun 9;11(1):2903. doi: 10.1038/s41467-020-16714-7. Nat Commun. 2020. PMID: 32518257 Free PMC article.
-
Silica Capsules Templated from Metal-Organic Frameworks for Enzyme Immobilization and Catalysis.Langmuir. 2021 Mar 16;37(10):3166-3172. doi: 10.1021/acs.langmuir.1c00065. Epub 2021 Mar 2. Langmuir. 2021. PMID: 33651618
-
Functional Capsules via Subcomponent Self-Assembly.Acc Chem Res. 2018 Oct 16;51(10):2423-2436. doi: 10.1021/acs.accounts.8b00303. Epub 2018 Sep 12. Acc Chem Res. 2018. PMID: 30207688
-
Cascade reactions catalyzed by metal organic frameworks.ChemSusChem. 2014 Sep;7(9):2392-410. doi: 10.1002/cssc.201402148. Epub 2014 Jul 31. ChemSusChem. 2014. PMID: 25082205 Review.
-
Integration of metal organic frameworks with enzymes as multifunctional solids for cascade catalysis.Dalton Trans. 2020 Aug 18;49(32):11059-11072. doi: 10.1039/d0dt02045a. Dalton Trans. 2020. PMID: 32808625 Review.
Cited by
-
Three-Stage Conversion of Chemically Inert n-Heptane to α-Hydrazino Aldehyde Based on Bioelectrocatalytic C-H Bond Oxyfunctionalization.ACS Catal. 2023 Jan 6;13(1):563-572. doi: 10.1021/acscatal.2c04003. Epub 2022 Dec 22. ACS Catal. 2023. PMID: 36644649 Free PMC article.
References
-
- Leveson-Gower R. B.; Mayer C.; Roelfes G. The Importance of Catalytic Promiscuity for Enzyme Design and Evolution. Nat. Rev. Chem. 2019, 3, 687–705. 10.1038/s41570-019-0143-x. - DOI
-
- Chen K.; Arnold F. H. Engineering New Catalytic Activities in Enzymes. Nat. Catal. 2020, 3, 203–213. 10.1038/s41929-019-0385-5. - DOI
-
- Dhakshinamoorthy A.; Asiri A. M.; Garcia H. Metal–Organic Frameworks as Multifunctional Solid Catalysts. Trends Chem. 2020, 2, 454–466. 10.1016/j.trechm.2020.02.004. - DOI
LinkOut - more resources
Full Text Sources
